噻吩取代喹喔啉供体-受体染料:合成、核磁共振光谱学、X 射线晶体学和光物理性质
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
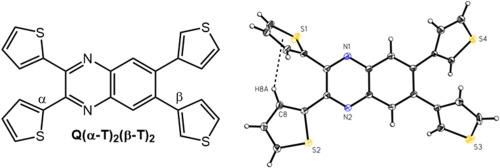
对含有供体-受体单元的荧光分子进行结构-性质关系研究,对于设计定制应用的有机材料非常重要。我们报告了一系列具有不同取代基位置的新型噻吩取代喹喔啉供体-受体染料的合成和表征。这些染料是采用微波辅助方法,通过一个或两个步骤从市售前体中合成的。在溶液中,质子核磁共振光谱研究显示,与单取代类似物相比,邻二取代喹喔啉衍生物中某些噻吩环质子有明显的上场偏移。这种上场偏移是由于立体拥塞导致噻吩-喹喔啉 C-C 键的旋转受限,从而导致某些质子被邻近的芳香环屏蔽。在固态下,对邻位二取代衍生物进行的单晶 X 射线衍射研究表明,它们表现出噻吩环的无序性。这些染料在溶液中具有微弱的发射性,在不同极性的溶剂中使用紫外-可见光和荧光光谱对其进行光物理表征,发现它们具有轻微到中等程度的发射溶解色度,Lippert-Mataga 分析估计的Δμ 值在 3 到 6 D 之间。噻吩环连接在喹喔啉环苯侧的染料比噻吩环连接在吡嗪侧的染料具有更高的溶色性,这说明了取代基位置对分子光物理特性的影响。DFT 和 TD-DFT 计算结果与观察到的染料光物理特性和电子结构相一致,有助于合理解释这些趋势。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Thiophene-substituted quinoxaline donor-acceptor dyes: Synthesis, NMR spectroscopy, X-ray crystallography, and photophysical properties
Structure-property relationship studies of fluorescent molecules containing donor-acceptor units are important for informing the design of organic materials for custom applications. We report the synthesis and characterization of a series of novel thiophene-substituted quinoxaline donor-acceptor dyes with varied substituent positioning. The dyes were synthesized using microwave-assisted methods in one or two steps from commercially available precursors. In solution, proton NMR spectroscopy studies revealed significant upfield shifts for certain thiophene ring protons in the o-disubstituted quinoxaline derivatives compared to their monosubstituted analogs. The upfield shifts resulted from restricted rotation about the thiophene-quinoxaline C–C bonds due to steric congestion leading to some protons being shielded by neighboring aromatic rings. In the solid state, single-crystal X-ray diffraction studies of the o-disubstituted derivatives showed that they exhibit thiophene ring disorder. These dyes are weakly emissive in solution, and their photophysical characterization using UV–vis and fluorescence spectroscopy in solvents of varying polarity revealed slight to moderate emission solvatochromism, with Lippert-Mataga analysis estimated Δμ values between 3 and 6 D. The dyes with thiophene rings attached to the benzene side of the quinoxaline ring exhibited a higher degree of solvatochromism than those with thiophene rings attached to the pyrazine side, illustrating the impact that substituent positioning can have on a molecule's photophysical properties. DFT and TD-DFT computational results were consistent with the observed photophysical properties and electronic structures of the dyes and aided in rationalization of the trends.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


