利用多物理场建模理解氧化还原液流电池的特征电化学阻抗谱数据
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
摘要
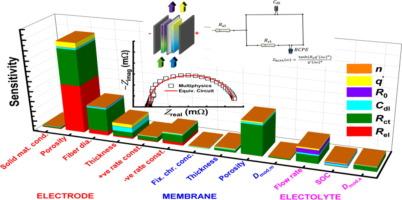
电化学阻抗光谱(EIS)是一种强大的表征方法,可用于探测电化学系统中普遍存在的(电)化学过程。尽管 EIS 在燃料电池研究中得到了广泛应用,但在氧化还原液流电池系统中的应用,尤其是简化的双电极全电池配置中的应用,则较为有限。在此,我们尝试将等效电路建模与经过验证的多物理场模型相结合,在频域流体力学条件下进行分析,以加强对钒氧化还原液流电池特征 EIS 数据的理解。在强调了氧化还原液流电池中 EIS 的系统线性和稳定性问题之后,我们特别使用我们的组合方法来研究不同电池组件特性对观察到的电静态 EIS 光谱和伴随的拟合等效电路元件参数的影响。对于所研究的两侧电极材料相同的双电极全电芯液流电池配置,观察到 EIS 光谱数据在光谱的不同端点由不同的质量或电荷传输过程主导。对获得的 EIS 光谱数据和拟合电路元件参数进行的灵敏度分析表明,电极形态特性、隔膜孔隙率和电解液流入条件主要决定了 EIS 光谱数据。从本文所做的分析类型中获得的启示可促进液流电池电池/电池组的诊断和有针对性的性能改进工作。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Understanding characteristic electrochemical impedance spectral data of redox flow batteries with multiphysics modeling
Electrochemical impedance spectroscopy (EIS) is a robust characterization method to probe prevalent (electro)chemical processes in an electrochemical system. Despite its extensive utilization in fuel cell research, the application of EIS in redox flow battery systems particularly for simplified two-electrode full-cell configurations is more limited. Herein we attempt to strengthen the understanding of characteristic EIS data of vanadium redox flow batteries by a combination of equivalent circuit modeling with a validated Multiphysics model analyzed under hydrodynamic conditions in frequency domain. Following a highlight of system linearity and stability concerns for EIS in redox flow batteries, we specifically use our combinatory approach to investigate the effects of different cell component properties on observed galvanostatic EIS spectra and accompanying fitted equivalent circuit element parameters. For the investigated two-electrode full-cell flow battery configuration with the same electrode material on both sides, the EIS spectral data is observed to be dominated by different mass or charge transport processes at different ends of the spectrum. Sensitivity analyses of both obtained EIS spectral data and fitted circuit elements parameters show that electrode morphological properties, membrane porosity, and electrolyte inflow conditions predominantly define the EIS spectral data. Insights from the type of analyses performed herein can facilitate flow battery cell/stack diagnostics and targeted performance improvement efforts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


