人工合成的 Kusunokinin 类似物对人类癌细胞株的抗癌活性
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
合成了一系列外消旋草乌甲素衍生物,并研究了它们对乳腺癌(MCF-7、MDA-MB468)、结肠癌(HT-29)、胆管癌(KKU-M213)和卵巢癌(A2780)等癌细胞的细胞毒活性和细胞活力。结果表明,化合物 6aa、6da 和 6de 对乳腺癌(MDA-MB468)、胆管癌(KKU-M213)、结肠癌(HT-29)和卵巢癌(A2780)细胞具有生长抑制作用,IC50 值(μM)分别为 13.分别为 13.77 ± 0.38、7.94 ± 0.45 和 4.22 ± 0.13(MDA-MB468);4.21 ± 0.21、0.97 ± 0.03 和 0.09 ± 0.02(KKU-M213);22.66 ± 0.23 和 15.62 ± 0.06(HT-29);13.11 ± 0.37、11.51 ± 0.43 和 1.87 ± 0.01(A2780)。有趣的是,阳性对照多柔比星对胆管癌 KKU-M213 和卵巢癌 A2780 细胞的细胞毒性低于 6da 和 6de。此外,这三种合成化合物对正常细胞 MMNK-1、Vero 和 L-929 的毒性也低于多柔比星。在与 CSF1R 的结合可能性方面,6de(-11.59 kcal/mol)和反式-(-)-kusunokinin(-11.75 kcal/mol)的对接能量和对接姿势都相似。6de 与 Trp550 通过 π-π 堆积相互作用,与反式-(-)-库苏诺金和反式-(+)-库苏诺金的作用方式相似。本文章由计算机程序翻译,如有差异,请以英文原文为准。
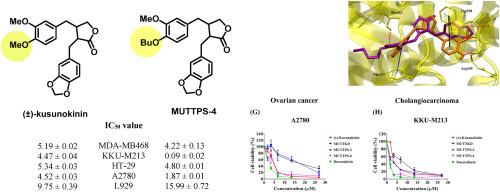
Anticancer activity of the synthetic kusunokinin analogues on human cancer cell lines
The series of racemic kusunokinin derivatives were synthesized and their cytotoxic activities and cell viability on cancer cells including breast cancer (MCF-7, MDA-MB468), colon cancer (HT-29), cholangiocarcinoma (KKU-M213) and ovarian cancer (A2780) cells were investigated. The results showed that compounds 6aa, 6da, and 6de exhibited growth inhibition against breast cancer (MDA-MB468), cholangiocarcinoma (KKU-M213), colon cancer (HT-29), and ovarian cancer (A2780) cells with IC50 values (μM) 13.77 ± 0.38, 7.94 ± 0.45, and 4.22 ± 0.13 (MDA-MB468); 4.21 ± 0.21, 0.97 ± 0.03, and 0.09 ± 0.02 (KKU-M213); 22.66 ± 0.23, and 15.62 ± 0.06 (HT-29); 13.11 ± 0.37, 11.51 ± 0.43, and 1.87 ± 0.01 (A2780); respectively. Interestingly, a positive control, doxorubicin, showed less cytotoxicity than 6da and 6de on cholangiocarcinoma KKU-M213 and ovarian cancer A2780 cells. Moreover, these three synthetic compounds also exhibited less toxicity than doxorubicin on the normal cells, MMNK-1, Vero and L-929. The binding possibility towards CSF1R, 6de (−11.59 kcal/mol) and trans-(−)-kusunokinin (−11.75 kcal/mol) were similar in both docking energies and docking poses. 6de interacted with Trp550 via π-π stacking in the similar manner with trans-(−)-kusunokinin and trans-(+)-kusunokinin.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


