基于后合成 MOF 纳米片检测质子载体在不同相对湿度下的传导特性
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
探索质子载流子在不同相对湿度(RH)下的传输能力对于合理设计和开发高性能质子交换膜(PEM)至关重要。然而,与湿度高度相关的传输通道和随机载流子分布使大多数膜材料不合格。在此,我们通过后合成配体交换制备了一系列具有稳定、可量化孔隙结构和不同导电基团的 MIL-53 金属有机框架(MOF)纳米片,然后通过旋涂组装了片状膜。我们证明,在低相对湿度条件下,基于载流子机制,载流子基团与水分子之间适当的结合能有利于质子转移。特别是,即使载体具有较高的质子解离常数,但强大的结合能也会捕获水分子,阻碍质子转移。因此,在 20% 相对湿度和 80 °C 条件下,AlBDC-COOH 的质子传导率为 13.6 mS cm-1,高于 AlBDC-SO3H(11.9 mS cm-1)。相反,随着水含量的增加,水分子的可用扩散空间逐渐减少,导致扩散能力降低,从而降低了载体转移的贡献。因此,跃迁转移成为主要的质子传导过程,而 AlBDC-SO3H 膜中丰富的氢键网络提供了更多的质子转移途径,因此在 80 °C 和 100% 相对湿度条件下,质子传导率高达 73.1 mS cm-1,比原始 Al-BDC 膜(6.2 mS cm-1)高出一个数量级以上。这项研究可为针对不同操作条件的 PEM 的官能团选择提供启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
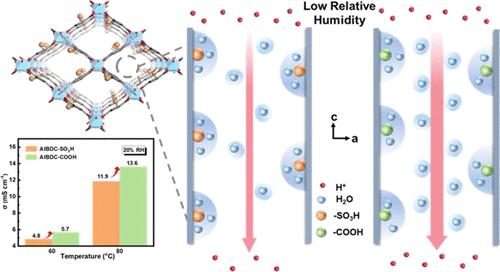
Detecting the Conduction Property of Proton Carriers at Different Relative Humidity Based on Postsynthetic MOF Nanosheets
Exploring the transfer ability of proton carriers at different relative humidity (RH) is vital for the rational design and development of high-performance proton exchange membranes (PEMs). However, the highly humidity-dependent transfer channel and random carrier distribution disqualify most membrane materials. Herein, a series of MIL-53 metal–organic framework (MOF) nanosheets with stable, quantifiable pore structures and different conducting groups are prepared through postsynthetic ligand exchange, followed by spin coating to assemble lamellar membranes. We demonstrated that proper binding energy between the carrier group and water molecule is favorable for proton transfer based on the vehicle mechanism at low RH. Particularly, strong binding energy traps water molecules, hindering proton transfer even though the carrier possesses a higher proton dissociation constant. Thus, at 20% RH and 80 °C, AlBDC–COOH attains a higher proton conductivity of 13.6 mS cm–1 than AlBDC–SO3H (11.9 mS cm–1). In contrast, with an incremental content of water, the available diffusion space of water molecules progressively diminishes, leading to a reduced diffusion ability and thus a lower contribution of vehicle transfer. Accordingly, jump transfer becomes the dominant proton conduction process, and the abundant hydrogen bond networks in the AlBDC–SO3H membrane provide more proton transfer paths and thus a higher proton conductivity of 73.1 mS cm–1 at 80 °C and 100% RH, over 1 order of magnitude higher than that of the pristine Al-BDC membrane (6.2 mS cm–1). This study may shed light on the functional group selection of PEMs targeting different operation conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


