来自脑炎患者的多种抗NMDAR自身抗体
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
在抗NMDAR脑炎患者中发现了针对谷氨酸能N-甲基-d-天冬氨酸受体(NMDAR)的自身抗体。两项研究显示,患者来源的自身抗体在表位结合和对 NMDAR 的作用模式上具有多样性,这为了解自身抗体诱导 NMDAR 功能低下背后的机制提供了启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
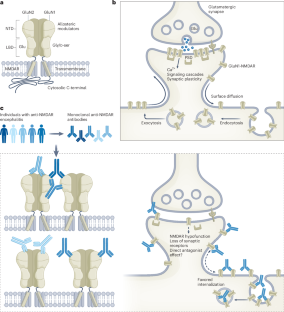

Diverse anti-NMDAR autoantibodies from individuals with encephalitis
Autoantibodies targeting glutamatergic N-methyl-d-aspartic acid receptors (NMDARs) are found in people with anti-NMDAR encephalitis. Two studies reveal that patient-derived autoantibodies are diverse in their epitope binding and modes of action on the NMDAR, providing insights into the mechanisms behind autoantibody-induced NMDAR hypofunction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


