低氧诱导因子 2α 通过调节磷脂代谢促进干型 Th2 细胞致病性极化
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
T 辅助细胞 2(Th2)可协调免疫力,抵御寄生虫感染,促进组织修复,但也会导致哮喘和组织纤维化等病症。在这里,我们研究了 Th2 细胞致病分化的驱动机制。对哮喘和慢性鼻炎患者的 CD4+ T 细胞进行的单细胞分析表明,Th2 细胞中缺氧诱导因子(HIF)2α 的表达量很高。在小鼠中,HIF2α的缺乏会影响Th2分化,并减轻哮喘炎症。单细胞和细胞系追踪方法勾勒出了从TCF1+Ly108+干样Th2细胞到ST2+CD25+致病性祖细胞的分化轨迹、HIF2α-GATA3回路通过肌醇多磷酸激酶(IPMK)的转录调控调节磷脂代谢和T细胞受体(TCR)-磷脂酰肌醇3-激酶(PI3K)-蛋白激酶B(AKT)的激活。IPMK在HIF2α缺陷细胞中的过表达促进了磷脂酰肌醇(3,4,5)-三磷酸(PIP3)的合成和致病性Th2细胞的分化,而药物抑制HIF2α则会抑制Th2细胞的致病性分化并减轻气道炎症。我们的研究结果让人们深入了解了促进 Th2 细胞介导的病理学的背景线索,并建议将 HIF2α 作为哮喘的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
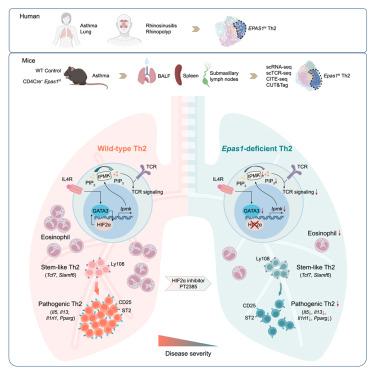
Hypoxia-inducible factor 2α promotes pathogenic polarization of stem-like Th2 cells via modulation of phospholipid metabolism
T helper 2 (Th2) cells orchestrate immunity against parasite infection and promote tissue repair but promote pathology in asthma and tissue fibrosis. Here, we examined the mechanisms driving pathogenic differentiation of Th2 cells. Single-cell analyses of CD4+ T cells from asthma and chronic rhinosinusitis patients revealed high expression of the hypoxia-inducible factor (HIF)2α in Th2 cells. In mice, HIF2α deficiency impaired Th2 differentiation and alleviated asthmatic inflammation. Single-cell and lineage tracing approaches delineated a differentiation trajectory from TCF1+Ly108+ stem-like Th2 cells to the ST2+CD25+ pathogenic progeny, depending on a HIF2α-GATA3 circuit that modulated phospholipid metabolism and T cell receptor (TCR)-phosphatidylinositol 3-kinase (PI3K)-protein kinase B (AKT) activation via transcriptional regulation of the inositol polyphosphate multikinase (IPMK). Overexpression of IPMK in HIF2α-deficient cells promoted Phosphatidylinositol (3,4,5)-trisphosphate (PIP3) synthesis and pathogenic Th2 cell differentiation, whereas pharmacological inhibition of HIF2α impaired pathogenic differentiation of Th2 cells and mitigated airway inflammation. Our findings provide insight into the contextual cues that promote Th2-mediated pathology and suggest HIF2α as a therapeutic target in asthma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


