远紫外辐射下过氧乙酸活化增强:微污染物降解和自由基评估
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
传统的紫外线/过乙酸工艺(UV/PAA)受到能量利用的限制。本文建立了一种高效的高级氧化工艺(AOP),利用波长为 222 纳米的远紫外光激活 PAA(UV222/PAA)来降解微污染物。UV222/PAA体系能有效降解多种微污染物,在300 s内,萘普生(NAP)在UV222/PAA体系中的降解率分别是在UV222/H2O2、UV222和UV254/PAA体系中的2.61倍、3.50倍和4.92倍。经测定,在 pH 值为 5.0 和 pH 值为 9.6 时,UV222 照射下分子态 PAA(PAA0)和离解态 PAA(PAA-)光解的先天量子产率分别为 0.51 和 0.84 摩尔爱因斯坦-1。根据已建立的动力学模型,乙酰过氧自由基(CH3(O)OO-)和乙酰氧基自由基(CH3(O)O-)与 NAP 的二阶速率常数分别为 1.74 × 107 和 1.08 × 106 M-1s-1。UV222、羟基自由基(HO-)和 CH3(O)OO- 是降解 NAP 的主要成分,它们的相对贡献率分别为 33.00 %、8.15 % 和 58.83 %。pH 值的增加对 NAP 降解有促进作用,在 UV222/PAA 系统中,自由基的稳态浓度随 pH 值的增加而增加。Cl-和HCO3-对NAP降解的影响不明显,而HA则抑制NAP的降解。在产物鉴定和理论计算分析的基础上,提出了 NAP 的降解途径。最后,还在真水中验证了 UV222/PAA 系统降解 NAP 的有效性。这项研究开发了一种高效的 PAA 活性 AOP,具有较高的自由基产率,可用于实际应用中的微污染物降解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
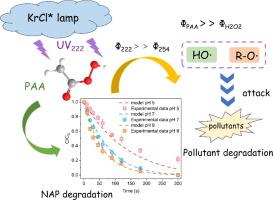
Enhanced peracetic acid activation under Far-UVC Radiation: Micropollutant degradation and radical assessment
Traditional ultraviolet/peracetic acid process (UV/PAA) was limited by energy utilization. Herein, an efficient advanced oxidation process (AOP) for activating PAA by the far-ultraviolet of 222 nm (UV222/PAA) was established for micropollutant degradation. UV222/PAA system can effectively degrade various kinds of micropollutants, and the degradation rate of naproxen (NAP) in UV222/PAA system were about 2.61, 3.50 and 4.92 times higher than that in UV222/H2O2, UV222 and UV254/PAA systems within 300 s, respectively. The innate quantum yields of molecular state PAA (PAA0) and dissociated state PAA (PAA−) photolysis under UV222 irradiation were determined to be 0.51 and 0.84 mol Einstein−1 at pH 5.0 and pH 9.6, respectively. Based on an established kinetic model, the second-order rate constants of acetylperoxyl radical (CH3(O)OO•) and acetyloxyl radical (CH3(O)O•) with NAP were determined to be 1.74 × 107 and 1.08 × 106 M−1s−1, respectively. UV222, hydroxyl radical (HO•) and CH3(O)OO• were mainly responsible for the degradation of NAP, and their relative contributions were 33.00 %, 8.15 %, and 58.83 %, respectively. The increase of pH showed a promoting effect on NAP degradation, and the steady-state concentration of radicals increased with the increase of pH in UV222/PAA system. Cl– and HCO3– have inconspicuous impact on NAP degradation, while HA inhibits the degradation of NAP. The degradation pathway of NAP was proposed on the basis of product identification and theoretical calculation analysis. Finally, the effectiveness of the UV222/PAA system for NAP degradation was also verified in real water. This work develops an efficient PAA activation AOP with high radical yield for micropollutant degradation in practical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


