通过硝酸氧化法对聚苯乙烯树脂及其一次性使用产品进行化学回收:其他塑料污染的机理和影响
IF 4.1
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
聚苯乙烯(PS)是一种广泛使用的塑料,尤其是在一次性使用的物品中,导致了大量废物的积累和严重的环境问题。解决这一问题的一个可行办法是采用先进的回收工艺来循环利用这种材料。本研究调查了通过硝酸氧化法对 PS 树脂(GP110)和商用 PS 产品进行化学回收的情况。此外,还研究了 PS 中的污染物 HDPE、PP 和 PVC 对反应机理和产品成分的影响。在 50 mL 水热高压釜中以 150-190 °C 的温度进行的小规模反应表明,GP110 被氧化并大部分转化为苯甲酸 (BA)、4-硝基苯甲酸 (4NB) 和 3-硝基苯甲酸 (3NB),反应时间短于 8 小时。与 GP110 树脂(∼42%)相比,商用 PS 产品(即勺子、盒子和杯子)在 170°C 下反应 4 小时后的碳回收率(∼24-29%)要低得多。由于这些产品是由摩尔质量较低的 PS 制成的,因此需要短于 4 小时的反应时间来减少碳的损失。在更大规模的反应(即 250 mL 水热反应器)中,将 SB 作为 PS 废弃物模型进行氧化,以评估将该工艺用于 PS 废弃物化学回收的可行性。该工艺(170 和 180 °C,3 小时)的碳回收率为 53-80%。根据反应条件和反应器的压力限制,固体与水溶液(0.23 克/毫升硝酸)和 PS 与硝酸的合适比率分别为 1/12 至 1/16 克/毫升和 1/2.8 至 1/4.2 克/克。关于受到其他塑料污染的 PS 废料,结果表明,在解聚 PS 时应避免受到 PP 的污染,因为这会造成不利影响,特别是由于产生过量的气态产物而导致系统压力过高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
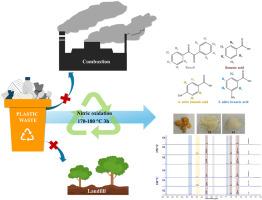
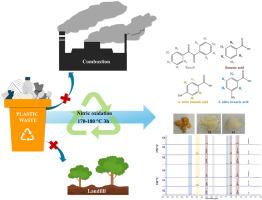
Chemical recycling of polystyrene resin and its single-used products via nitric acid oxidation: Mechanisms and effects of other plastic contaminations
Polystyrene (PS) is a widely used plastic, especially in single-use items, leading to a large accumulation of waste and serious environmental problems. A promising solution to this is an advanced recycling process to circularly use the material. In this study, the chemical recycling of PS resin (GP110) and commercial PS products via nitric acid oxidation is investigated. In addition, the effects of HDPE, PP, and PVC as contaminants in PS on the reaction mechanisms and product compositions are examined. Small-scaled reactions at 150–190 °C in a 50 mL hydrothermal autoclave revealed that GP110 is oxidized and mostly converted to benzoic acid (BA), 4-nitrobenzoic acid (4NB), and 3-nitrobenzoic acid (3NB) with a reaction time shorter than 8 h. The conversion of BA to 3NB is more favorable at high temperatures. The reactions at 170 °C for 4 h on commercial PS products, i.e., spoons, boxes, and cups, led to a much lower carbon recovery (∼24–29 %) than GP110 resin (∼42 %). A reaction time shorter than 4 h is required to reduce carbon loss, as these products are fabricated from lower molar mass PS. The oxidation of SB as a model PS waste in a larger scaled reaction, i.e., a 250 mL hydrothermal reactor, is used to assess the feasibility of applying the process for chemical recycling of PS waste. The processes (at 170 and 180 °C for 3 h) provided 53–80 % carbon recovery. The suitable ratios of solid to aqueous solution (0.23 g/mL nitric) and PS to nitric acid are in the range of ∼1/12 to 1/16 g/mL and 1/2.8 to 1/4.2 g/g, respectively, depending on the reaction conditions and the pressure limitation of the reactor. Concerning PS waste contaminated with other plastics, the results suggest that contamination with PP should be avoided in depolymerizing PS, as this can cause adverse effects, especially excessively built-up pressure in the system due to the generation of excess gaseous products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer
化学-高分子科学
CiteScore
7.90
自引率
8.70%
发文量
959
审稿时长
32 days
期刊介绍:
Polymer is an interdisciplinary journal dedicated to publishing innovative and significant advances in Polymer Physics, Chemistry and Technology. We welcome submissions on polymer hybrids, nanocomposites, characterisation and self-assembly. Polymer also publishes work on the technological application of polymers in energy and optoelectronics.
The main scope is covered but not limited to the following core areas:
Polymer Materials
Nanocomposites and hybrid nanomaterials
Polymer blends, films, fibres, networks and porous materials
Physical Characterization
Characterisation, modelling and simulation* of molecular and materials properties in bulk, solution, and thin films
Polymer Engineering
Advanced multiscale processing methods
Polymer Synthesis, Modification and Self-assembly
Including designer polymer architectures, mechanisms and kinetics, and supramolecular polymerization
Technological Applications
Polymers for energy generation and storage
Polymer membranes for separation technology
Polymers for opto- and microelectronics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


