在急性疼痛动物模型中,阿片能受体和巴氏核中的 D1 类多巴胺受体对疼痛相关行为的交互作用。
IF 2.5
3区 心理学
Q1 BEHAVIORAL SCIENCES
引用次数: 0
摘要
阿片能系统和多巴胺能系统在处理脑内核(NAc)疼痛信息时发挥着重要作用。本研究探讨了 NAc 区域的阿片能和 D1 类多巴胺受体之间的相互作用可能影响急性疼痛相关行为的假设。160 只成年雄性 Wistar 大鼠单侧接受了不同剂量的药物溶液或载体。首先,各组动物分别接受了不同剂量的吗啡(5、10和25毫摩尔/0.5微升)和不同剂量的SKF38393(1.5、3、6和12毫摩尔/0.5微升),它们分别是NAc区域的阿片和D1样受体激动剂。在第二组实验中,动物在接受有效剂量的吗啡(10 毫摩尔/0.5 微升)之前,分别摄入不同剂量(1.5、3、6 和 12 毫摩尔/0.5 微升)的 D1 样受体拮抗剂 SCH23390。在最后一项实验中,先给动物注射纳洛酮(1.5、5 和 15 毫摩尔/0.5 μL),然后再注射有效剂量的 SKF38393(3 毫摩尔/0.5 μL)。然后用尾弹试验测量它们的急性痛阈值。主要研究结果表明,单独在NAc内注射吗啡和SKF38393会引起抗痛觉反应。然而,SCH23390的累积体内注射可显著降低NAc内注射吗啡引起的抗痛觉反应。此外,NAc 内注射纳洛酮也会显著降低NAc 内注射 SKF38393 所引起的抗痛觉反应。有趣的是,与纳洛酮逆转 SKF38393 的镇痛效果(η2 = 0.49)相比,SCH23390 逆转吗啡的镇痛效果(η2 = 0.61)更为有效。研究结果表明,NAc 中的阿片能系统和多巴胺系统共同产生镇痛效果。这一观点有可能提高低剂量阿片类药物的镇痛效果,最终减少其在未来临床中的使用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
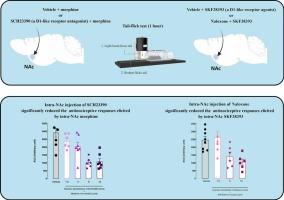
The interaction effects between opioidergic and D1-like dopamine receptors in the nucleus accumbens on pain-related behaviors in the animal model of acute pain
The opioidergic and dopaminergic systems play an essential role in processing pain information in the nucleus accumbens (NAc). The present work examined the hypothesis that interaction between opioidergic and D1-like dopamine receptors in the NAc area may influence acute pain-related behaviors. One hundred sixty adult male Wistar rats unilaterally received different doses of the drug solution or vehicle. First, separate groups of animals received different doses of morphine (5, 10, and 25 mmol/0.5 μL) and various doses of SKF38393 (1.5, 3, 6, and 12 mmol/0.5 μL) as opioid and D1-like receptor agonists in the NAc region, respectively. In the second set of experiments, animals got different amounts (1.5, 3, 6, and 12 mmol/0.5 μL) of SCH23390, a D1-like receptor antagonist, before an effective dose of morphine (10 mmol/0.5 μL). In the last experiment, the animals were given naloxone (1.5, 5, and 15 mmol/0.5 μL) before they were given an effective dose of SKF38393 (3 mmol/0.5 μL). The tail-flick test was then used to measure their acute pain threshold. The main findings showed that intra-NAc injection of morphine and SKF38393 alone causes antinociceptive responses. However, the intra-accumbal injection of SCH23390 significantly reduced the antinociceptive responses elicited by intra-NAc morphine. Additionally, intra-NAc naloxone significantly reduced the antinociceptive effects elicited by intra-NAc SKF38393. Interestingly, SCH23390 was more effective in reversing the analgesic effects of morphine (η2 = 0.61) than naloxone in reversing the analgesic effects of SKF38393 (η2 = 0.49). The findings suggest that the opioidergic and dopamine systems in the NAc collaborate to produce pain-relieving effects. This insight could potentially enhance the effectiveness of lower doses of opioids for pain management, ultimately reducing their usage in clinical settings in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.40
自引率
2.80%
发文量
122
审稿时长
38 days
期刊介绍:
Pharmacology Biochemistry & Behavior publishes original reports in the areas of pharmacology and biochemistry in which the primary emphasis and theoretical context are behavioral. Contributions may involve clinical, preclinical, or basic research. Purely biochemical or toxicology studies will not be published. Papers describing the behavioral effects of novel drugs in models of psychiatric, neurological and cognitive disorders, and central pain must include a positive control unless the paper is on a disease where such a drug is not available yet. Papers focusing on physiological processes (e.g., peripheral pain mechanisms, body temperature regulation, seizure activity) are not accepted as we would like to retain the focus of Pharmacology Biochemistry & Behavior on behavior and its interaction with the biochemistry and neurochemistry of the central nervous system. Papers describing the effects of plant materials are generally not considered, unless the active ingredients are studied, the extraction method is well described, the doses tested are known, and clear and definite experimental evidence on the mechanism of action of the active ingredients is provided.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


