通过盐酸羟胺催化 C(S)-N 键裂解和形成的伯氨基硫代酰胺与伯胺和仲胺的反式酰胺化反应†。
IF 2.5
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
盐酸羟胺通过 C(S)-N 键的裂解和形成,催化了伯硫酰胺与伯胺和仲胺的反氨化反应。使用绿色、良性的盐酸羟胺作为催化剂,将现成的伯硫酰胺作为底物,转化为所需的仲硫酰胺和叔硫酰胺。这种方法具有优异的官能团耐受性和广泛的底物范围,有望广泛应用于合成化学、药物化学等领域。本文章由计算机程序翻译,如有差异,请以英文原文为准。
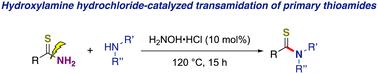
Transamidation of primary thioamides with primary and secondary amines via C(S)–N bond cleavage and formation by hydroxylamine hydrochloride catalysis†
Hydroxylamine hydrochloride-catalyzed transamidation of primary thioamides with primary and secondary amines via C(S)–N bond cleavage and formation has been reported. Readily available primary thioamides are employed as substrates to convert desired secondary and tertiary thioamides using green and benign hydroxylamine hydrochloride as a catalyst. The utility of this approach has been demonstrated via excellent functional group tolerance and broad substrate scope, which is expected to be widely used in fields such as synthetic chemistry, pharmaceutical chemistry, etc.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

New Journal of Chemistry
化学-化学综合
CiteScore
5.30
自引率
6.10%
发文量
1832
审稿时长
2 months
期刊介绍:
A journal for new directions in chemistry
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


