芳基锍盐与芳基羧酸衍生物的可控叔胺促进光活化无金属羰基化反应†。
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
传统的过渡金属催化羰基化反应是将亲电试剂、CO 和亲核试剂直接加入高附加值产品的有力工具。尽管这些金属催化羰基化策略可以高效合成羰基化化合物,但无金属体系仍然是羰基化反应中一个极具吸引力的方向。受无金属自由基羰基化研究成果的启发,我们在此介绍一种利用光激发电子供体-受体(EDA)复合物进行芳基锍盐羰基化的光化学方法。这种方法不含金属,适用范围广,能以简化的方式获得多种芳基羧酸衍生物。值得注意的是,通过选择不同的胺,可以捕获反应中间体,然后逐步淬灭。它有望成为合成芳基羧酸衍生物的传统羰基化方法的直接绿色替代方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
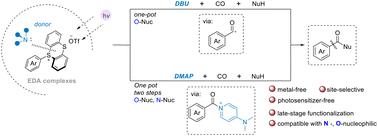
Controllable tertiary amine-promoted photoactivation metal-free carbonylation of aryl sulfonium salts to aryl carboxylic acid derivatives†
Conventional transition metal-catalyzed carbonylative reactions are a powerful tool for the direct incorporation of electrophilic reagents, CO, and nucleophilic reagents into high-value-added products. Although these metal-catalyzed carbonylation strategies can efficiently synthesize carbonylated compounds, metal-free systems remain an attractive direction in carbonylative reactions. Inspired by the achievements in metal-free radical carbonylation, herein we describe a photochemical method for the carbonylation of aryl sulfonium salts using photoexcitation of electron donor–acceptor (EDA) complexes. This strategy is metal-free and widely applicable, enabling ready access to a wide range of aryl carboxylic acid derivatives in a simplified manner. Notably, by choosing different amines, the reaction intermediates can be captured and then quenched stepwise. It has the potential to be a direct green alternative to conventional carbonylation methods for the synthesis of aryl carboxylic acid derivatives.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


