通过电子供体-受体复合物,以可见光驱动酮基硅烷烯醇醚的α-磺酰化反应†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
β-酮砜在药物和生物活性化合物中的多种用途引起了人们对其合成的浓厚兴趣。然而,现有的合成方法通常依赖于过渡金属催化剂,而过渡金属催化剂需要大量纯化,且产量较低。在此,我们提出了一种经济高效、不含金属和光催化剂的可见光电子供体-受体(EDA)复合物介导的磺酰化方法,该方法以硫代磺酸盐(受体)和 DABCO 作为电子供体,在温和的条件下对酮基硅烷烯醇醚进行磺酰化,提供了一种更高效、更直接的方法。我们的方法能够以良好的产率合成各种 β-酮砜衍生物,包括生物活性分子和晚期分子。与现有技术相比,我们的方法具有以下几个显著优势:(i) 无过渡金属和光氧化催化剂条件;(ii) 无需外部 SO2 源;(iii) 底物范围广;(iv) 副产品可回收和再利用;(v) 极佳的原子经济性、反应质量效率、过程质量强度以及 E 因子和 EcoScale 分数,突出了其效率和经济可持续性。详细的机理研究证实,EDA 复合物介导的自由基过程无需催化剂即可运行。本文章由计算机程序翻译,如有差异,请以英文原文为准。
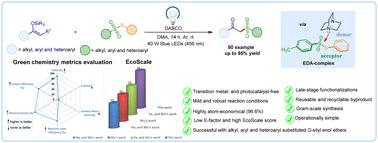
Visible light-driven α-sulfonylation of ketone-derived silyl enol ethers via an electron donor–acceptor complex†
The diverse utility of β-ketosulfones in pharmaceuticals and bioactive compounds has generated considerable interest in their synthesis. However, existing synthetic approaches often depend on transition-metal catalysts, which require extensive purification and result in low yields. Herein, we present a cost-effective, metal- and photocatalyst-free, visible light electron donor–acceptor (EDA) complex-mediated sulfonylation of ketone-derived silyl enol ethers with thiosulfonates (acceptor) and DABCO as an electron donor under mild conditions, offering a more efficient and straightforward approach. Our method enables the synthesis of a diverse range of β-ketosulfone derivatives, including biologically active and late-stage molecules, in good yields. Our strategy offers several significant advantages over existing techniques, which include (i) transition-metal and photoredox catalyst-free conditions; (ii) no need for an external SO2 source; (iii) broad substrate scope; (iv) recyclable and reusable by-products; and (v) excellent atom economy, reaction mass efficiency, process mass intensity, and E-factor and EcoScale scores, highlighting its efficiency and economic sustainability. Detailed mechanistic studies confirm the involvement of an EDA-complex-mediated radical process that operates without a catalyst.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


