合成核糖-1-磷酸†的脱氨基生物催化级联反应
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
核糖-1-磷酸(Rib1P)是核苷磷酸化酶合成难以获得的核苷类似物的关键底物。然而,用传统方法制备核糖-1-磷酸时,由于产量低、选择性差,阻碍了核糖-1-磷酸在制备合成中的应用。虽然生物催化可以直接从天然核苷中获得 Rib1P,但这些转化过程受到严格的热力学控制,而且产量低、操作步骤繁琐。为了应对这些挑战,我们开发了一种生物催化级联法,可将天然鸟苷近乎完全地转化为α-异构纯 Rib1P。这条路线的关键是鸟嘌呤脱氨酶,它能去除累积的鸟嘌呤副产物。在优化的条件下,这种级联方法很容易扩展到克级规模,分离产率高达 79%,纯度高达 94%,无需任何层析。我们的级联方法减少了以往方法固有的有毒试剂和纯化步骤,减轻了该路线的环境负担,CHEM21 Zero Pass 和 E 因子计算也证实了这一点。因此,我们的工作将广泛加强核苷磷酸化酶介导化学的适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
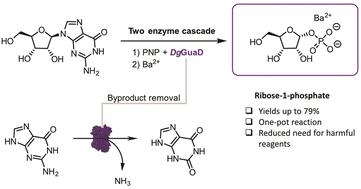
A deamination-driven biocatalytic cascade for the synthesis of ribose-1-phosphate†
Ribose-1-phosphate () is a key substrate for the synthesis of difficult-to-access nucleoside analogues by nucleoside phosphorylases. However, its use in preparative synthesis is hampered by low yields and low selectivity during its preparation by conventional methods. Although biocatalysis permits straightforward access to directly from natural nucleosides, these transformations are tightly thermodynamically controlled and suffer from low yields and non-trivial work-up procedures. To address these challenges, we developed a biocatalytic cascade that allows near-total conversions of natural guanosine into α-anomerically pure . The key to this route is a guanine deaminase, which removes the accumulated guanine byproduct. Under optimised conditions, this cascade proved readily scalable to the gram scale, delivering isolated yields of up to 79% and a purity of 94% without any chromatography. Our cascade approach reduced the need for toxic reagents and purification steps inherent to previous methods, reducing the environmental burden of the route, as confirmed by CHEM21 Zero Pass and E-factor calculations. Thus, our work will broadly strengthen the applicability of nucleoside phosphorylase-mediated chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


