产前抗生素会降低母乳中的 IgA,诱发小鼠后代菌群失调,增加新生儿对细菌性败血症的易感性
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
摘要
20%-30%的孕妇会服用抗生素(Abx),但人们对抗生素对新生儿免疫系统发育的影响知之甚少。我们的研究表明,服用过抗生素的母鼠所生的新生小鼠更容易患晚期败血症。这种易感性与较低的母体母乳免疫球蛋白 A (IgA)、新生儿粪便 IgA 和肠道细菌的 IgA 涂层有关,从而导致肠道病原菌的转移。经 Abx 处理的母亲所生的断奶后的年轻成人回肠和结肠中的 IgA+ 浆细胞、粪便分泌型 IgA(SIgA)、结肠 CD4+ T 调节性淋巴细胞和 T 辅助细胞 17 样淋巴细胞减少,粪便微生物群的多样性降低。然而,使用能恢复 SIgA 分泌的 apyrase 治疗可促进母乳中 IgA 的分泌,并保护幼崽免受败血症的影响。此外,未接受过治疗的母亲所产的母乳可挽救接受过 Abx 治疗的母亲所生幼崽的表型。我们的数据强调了产前 Abx 对母乳 IgA 的影响,以及它们对母乳喂养所介导的肠粘膜免疫功能的长期影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
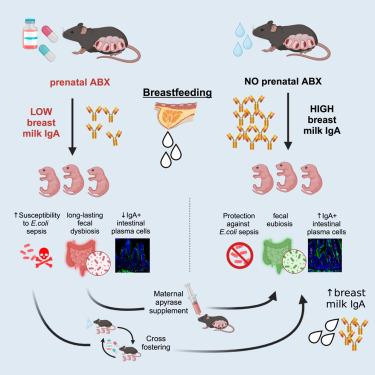
Prenatal antibiotics reduce breast milk IgA and induce dysbiosis in mouse offspring, increasing neonatal susceptibility to bacterial sepsis
Antibiotics (Abx) are administered to 20%–30% of pregnant women, but their effects on neonatal immune development are poorly understood. We show that newborn mice born to Abx-treated dams are more susceptible to late-onset sepsis. This susceptibility is linked to lower maternal breast milk immunoglobulin A (IgA), neonatal fecal IgA, and IgA coating of intestinal bacteria, thus causing the translocation of intestinal pathobionts. Weaned young adults born to Abx-treated mothers had reduced IgA+ plasma cells in the ileum and colon, fecal secretory IgA (SIgA), colonic CD4+ T regulatory lymphocytes and T helper 17-like lymphocytes, and a less diverse fecal microbiome. However, treatment with apyrase, which restores SIgA secretion, prompted IgA production in breast milk and protected pups from sepsis. Additionally, breast milk from untreated mothers rescued the phenotypes of pups born to Abx-treated mothers. Our data highlight the impact of prenatal Abx on breast milk IgA and their long-term influence on intestinal mucosal immune function mediated by breastfeeding.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


