与超高镍阴极耦合的基于 LaCl3 的固体电解质的界面降解
IF 9.1
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
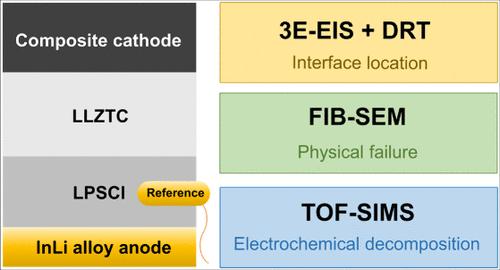
尽管氯化物固体电解质(SE)与高镍正极具有竞争兼容性,但在正极/SE界面上仍不可避免地会发生副反应,导致全固态锂电池(ASSLB)在循环过程中容量衰减。本文开发了一种三电极 ASSLB 测试装置,以全面揭示超高镍 LiNi0.92Co0.05Mn0.03O2 (NCM92) 正极与基于 LaCl3 的氯化物 SE Li0.447La0.475Zr0.059Ta0.179Cl3 (LLZTC) 配对的界面失效机制。弛豫时间分布(DRT)分析清楚地表明,ASSLB 的降解伴随着 NCM92/LLZTC 界面阻抗的显著增加,在较高的截止充电电压 4.8 V 对 Li+/Li 时,这种阻抗增加变得更加明显。此外,飞行时间二次离子质谱法(ToF-SIMS)和聚焦离子束扫描电子显微镜(FIB-SEM)分析也证实了活性晶格氧和 NCM92/LLZTC 界面物理接触丧失导致的劣化。这些研究结果表明,阴极/SE 界面的电化学降解和物理接触失效是导致 ASSLB 容量衰减的主要原因。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Interface Degradation of LaCl3-Based Solid Electrolytes Coupled with Ultrahigh-Nickel Cathodes
Despite competitive compatibility with high-nickel cathodes, chloride solid electrolytes (SEs) still experience inevitable side reactions at the cathode/SE interface, causing capacity decay in all-solid-state lithium batteries (ASSLBs) during cycling. Herein, a three-electrode ASSLB testing device is developed to comprehensively reveal the interface failure mechanisms of the ultrahigh-nickel LiNi0.92Co0.05Mn0.03O2 (NCM92) cathode paired with LaCl3-based chloride SE Li0.447La0.475Zr0.059Ta0.179Cl3 (LLZTC). Distribution of relaxation time (DRT) analysis clearly shows the ASSLB degradation accompanied by a significant NCM92/LLZTC interface impedance increase, which becomes more pronounced at the higher cutoff charging voltage of 4.8 V vs Li+/Li. Furthermore, time-of-flight secondary ion mass spectrometry (ToF-SIMS) and focused ion beam scanning electron microscopy (FIB-SEM) analysis also confirm the deterioration arising from active lattice oxygen and loss of physical contact at the NCM92/LLZTC interface. These findings reveal both electrochemical degradation and physical contact failure at the cathode/SE interface as key causes of the ASSLBs’ capacity decay.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


