螺旋状霍洛石纳米管中混合 CH4 物理吸附-水合物形成的分子洞察:对储能的影响
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
从微观上深入研究霍洛石纳米管(HNTs)中的混合 CH4 物理吸附-水合物形成,对于理解天然气在 HNTs 中的固化存储以及开发储能技术至关重要。本文通过吸附-水合混合(AHH)方法进行了大规模微秒经典分子动力学模拟,研究了HNTs中的CH4存储,揭示了气水比的影响。模拟结果表明,通过吸附-水合混合法,HNTs 是储存 CH4 的优良纳米材料。HNTs 内外的 CH4 物理吸附和水合物形成受 HNTs 表面性质和 CH4/H2O 分子动力学特性的深刻影响。HNT 外表面具有相对疏水性,可吸附 CH4 分子形成纳米气泡。此外,吸附在外表面的 CH4 分子被紧紧困在水合物固体和外表面之间,抑制了它们的动力学行为,有利于通过物理吸附储存 CH4。HNT 的内表面具有极强的亲水性,可强烈吸附 H2O 分子;因此,CH4 水合物可在 HNT 内部形成。与 HNT 外部相比,HNT 内部的 CH4 和 H2O 分子更难转化为水合物。适度的气水比有利于 CH4 的物理吸附和水合物的形成,而过高或过低的气水比则不利于 CH4 的有效储存。这些见解有助于开发高效的甲烷固化储存技术。本文章由计算机程序翻译,如有差异,请以英文原文为准。
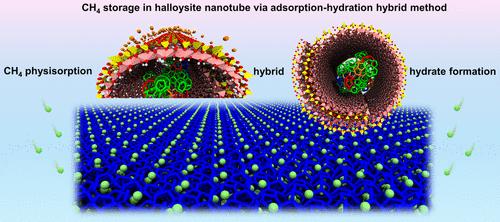
Molecular Insights into Hybrid CH4 Physisorption-Hydrate Formation in Spiral Halloysite Nanotubes: Implications for Energy Storage
A microscopic insight into hybrid CH4 physisorption-hydrate formation in halloysite nanotubes (HNTs) is vital for understanding the solidification storage of natural gas in the HNTs and developing energy storage technology. Herein, large-scale microsecond classical molecular dynamics simulations are conducted to investigate CH4 storage in the HNTs via the adsorption-hydration hybrid (AHH) method to reveal the effect of gas–water ratio. The simulation results indicate that the HNTs are excellent nanomaterials for CH4 storage via the adsorption-hydration hybrid method. The CH4 physisorption and hydrate formation inside and outside of the HNTs are profoundly influenced by the surface properties of the HNTs and the kinetic characteristics of CH4/H2O molecules. The outer surfaces of the HNTs exhibit relative hydrophobicity and adsorb CH4 molecules to form nanobubbles. Moreover, CH4 molecules adsorbed on the outer surface are tightly trapped between the hydrate solids and the outer surface, inhibiting their kinetic behavior and favoring CH4 storage via physisorption. The inner surface of the HNTs exhibits extreme hydrophilicity and strongly adsorbs H2O molecules; thus, CH4 hydrate can form inside of the HNTs. It is more difficult for CH4 and H2O molecules inside of the HNTs to convert into hydrates than for those outside of the HNTs. A moderate gas–water ratio is advantageous for CH4 physisorption and hydrate formation, whereas excessively high or low gas–water ratios are unfavorable for efficient CH4 storage. These insights can help to develop an efficient CH4 solidification storage technology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


