细胞样拥挤微环境中的酪氨酸酶氧化交联用于可见抑制剂筛选
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
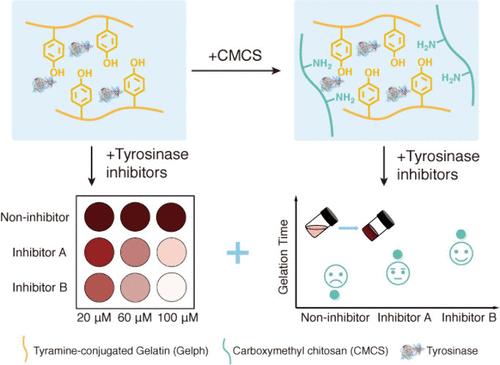
由于许多疾病和失调都是由功能失调或过度/表达不足的酶引起的,因此筛选药理相关酶的抑制剂已成为药物发现、临床诊断、酶工程和其他医学领域的一种有前途的工具。然而,尽管最近取得了一些进展,大多数抑制剂筛选仍通常在稀释介质中进行,浓度与酶实际存在的介质相差甚远,这可能导致药物在体内转化时失败。在这里,我们利用酪氨酸酶氧化交联水凝胶系统在体外构建了一个类似凝胶的细胞内生物环境,该环境更接近酶的真实催化环境。我们报告了一种简单有效的抑制剂评估策略,该策略可根据添加抑制剂会导致系统颜色变化和机械变化的原理,快速比较抑制剂的抑制强度。通过分子对接,我们进一步证明了抑制剂在不同浓度下的不同性能。通过在体外构建类似细胞的拥挤环境,该系统在新药开发方面展现出了诱人的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tyrosinase Oxidative Cross-Linking in the Cell-Like Crowded Microenvironment for Visible Inhibitor Screening
Owing to many diseases and disorders being caused by dysfunctional or over/underexpressed enzymes, screening for inhibitors of pharmacologically relevant enzymes has emerged as a promising tool for drug discovery, clinical diagnostics, enzyme engineering, and other medical fields. However, despite the recent advances, most inhibitor screenings are still usually conducted in dilute media, at concentrations far from the media in which the enzymes are actually found, which may cause drugs to fail when translated to in vivo. Herein, we build a gel-like intracellular biological environment in vitro using a tyrosinase oxidative cross-linking hydrogel system that is closer to the real catalytic environment of enzymes. We report a straightforward and effective inhibitor evaluation strategy that can quickly compare the inhibitory strengths of inhibitors based on the principle that adding inhibitors causes color changes and mechanical changes in the system. Enabled by molecular docking, we further demonstrate the different performances of the inhibitors at different concentrations. By construction of the cell-like crowded environment in vitro, this system shows an appealing application prospect for new drug development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


