在金属间 Cu1Pt 化合物上将 1,2-丙二醇无碱氧化成乳酸
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
聚乳酸(PLA)塑料是最受欢迎的可生物降解和生物相容性材料;然而,其单体乳酸(LA)目前是通过低效发酵工艺生产的。本文合成了活性炭支撑的铂铜金属间化合物,并将其用于 1,2-丙二醇(1,2-PDO)向 LA 的选择性氧化,而无需均相碱或碱性支撑物的参与。研究发现,斜方金属间 Cu1Pt 化合物表现出优异的性能,每个表面铂原子的计算周转频率达到 10 049 h-1。多种表征和 DFT 计算显示,铂铜金属间化合物对分子氧和 1,2-PDO 的吸附和/或活化具有活性。对吸附的 1,2-PDO 的拉曼分析进一步表明,1,2-PDO 与斜方体金属间化合物 Cu1Pt 的接触很强,然后末端羟基优先被氧化,从而促进了 LA 的形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
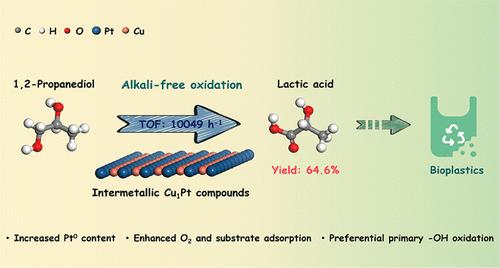
Alkali-Free Oxidation of 1,2-Propanediol to Lactic Acid over Intermetallic Cu1Pt Compounds
Polylactic acid (PLA) plastics are the most popular biodegradable and biocompatible materials; however, their monomer, lactic acid (LA), is currently produced via an inefficient fermentation process. Herein, activated carbon-supported Pt–Cu intermetallic compounds were synthesized and utilized in the selective oxidation of 1,2-propanediol (1,2-PDO) toward LA without the participation of homogeneous alkali or a basic support. It was found that rhombohedral intermetallic Cu1Pt compounds exhibited excellent performance and the calculated turnover frequency of each surface Pt atom reached 10 049 h–1. The yield of free LA reached 64.6% with a complete conversion of 1,2-PDO at 100 °C within 2 h. Diverse characterization and DFT calculations revealed that Pt–Cu intermetallic compounds were active for the adsorption and/or activation of molecular oxygen and 1,2-PDO. Raman analysis of adsorbed 1,2-PDO further disclosed that 1,2-PDO contacted with rhombohedral intermetallic Cu1Pt compounds strongly, and then the oxidation of the terminal hydroxyl group was performed preferentially, which promoted the formation of LA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


