MAZ 可调节少突胶质前体细胞的铁凋亡、凋亡和分化。
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
摘要
少突胶质细胞前体细胞(OPC)对脱髓鞘损伤反应迅速。然而,OPCs的细胞死亡可能会阻碍其拯救作用,从而导致不完全的再髓鞘化。本研究旨在探讨脱髓鞘小鼠体内MYC相关锌指蛋白(MAZ)的表达以及MAZ对OPC细胞死亡形式的影响。小鼠接受脱髓鞘药物(铜试剂,CZ)治疗5周。组织学染色显示,喂食 CZ 会引发胼胝体(CC)脱髓鞘。铁含量和铁突变标记物的检测表明,CZ处理过的小鼠的胼胝体发生了铁突变。值得注意的是,我们发现喂食 CZ 会导致 CC 中 MAZ 的表达减少。通过CCK-8测定、铁含量、MDA水平、GSH水平和铁败坏标记物检测、脂质ROS检测和4-HNE免疫荧光染色,我们发现敲除MAZ促进了OPC铁败坏。我们还评估了表达MAZ的OPC对铁突变诱导剂厄拉司汀的敏感性,结果表明表达MAZ的OPC对厄拉司汀诱导的铁突变具有抵抗力。TUNEL染色表明,敲除MAZ可抑制PI3K/Akt的激活,从而促进OPCs的凋亡。MBP的免疫荧光染色表明,敲除MAZ抑制了OPC的分化。此外,我们还阐明了MAZ对OPC死亡和分化具有保护作用的机制,这可能是通过SOX2的转录激活实现的。我们的研究结果介绍了MAZ对OPC存活和分化的有益调节作用,它可以作为脱髓鞘疾病的潜在治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
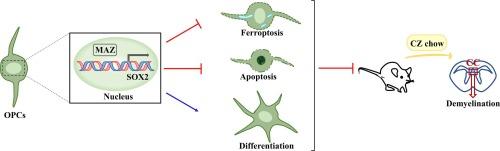
MAZ regulates ferroptosis, apoptosis and differentiation of oligodendrocyte precursor cells
Oligodendrocyte precursor cells (OPCs) respond rapidly to demyelination injury. However, the rescuing effects may be hindered by cell death of OPCs, leading to incomplete remyelination. This study aimed to explore the expression of MYC-associated zinc finger protein (MAZ) in demyelinated mice and the effects of MAZ on cell death form and differentiation of OPCs. Mice received demyelinating agent (cuprizone, CZ) for 5 weeks. Histological staining demonstrated that CZ feeding triggered demyelination in the corpus callosum (CC). Detection of iron content and ferroptosis markers indicated that ferroptosis was inducted in the CC of CZ-treated mice. Notably, we found CZ feeding resulted in a reduction of MAZ expression within the CC. Using CCK-8 assay, detection of iron content, MDA level, GSH level, and ferroptosis markers, lipid ROS detection, and immunofluorescence staining for 4-HNE, we found that knockdown of MAZ facilitated OPC ferroptosis. We also evaluated the susceptibility of MAZ-overexpressing OPCs to ferroptosis inducer, erastin, and demonstrated that MAZ-overexpressing OPCs were resistant to erastin-induced ferroptosis. TUNEL staining and western blot analysis indicated that MAZ knockdown promoted apoptosis of OPCs by inhibiting PI3K/Akt activation. Immunofluorescence staining of MBP indicated that knockdown of MAZ inhibited OPC differentiation. Moreover, we elucidate the mechanism responsible for MAZ’s protective effects on OPC death and differentiation, which may be achieved through transcriptional activation of SOX2. Our findings introduced MAZ as a beneficial modulator of OPC survival and differentiation, and it could serve as a potential therapeutic target for demyelination diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


