表没食子儿茶素没食子酸酯可在体外和体内抑制乳腺癌中的精氨酸甲基转移酶 5 和 Zeste 同源体增强子 2。
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
目的:组蛋白甲基转移酶是一种选择性甲基化组蛋白和非组蛋白上赖氨酸或精氨酸残基的酶,分为赖氨酸甲基转移酶和精氨酸甲基转移酶。值得注意的是,EZH2 和 PRMT5 分别催化 H3 在 K27 处的三甲基化和 H4 在 R3 处的对称二甲基化。这些甲基化事件被认为是癌症中特有的组蛋白抑制标记。据报道,PRMT5和EZH2在多种癌症中过度表达,并被认为是一种药物靶点。本研究旨在探索表没食子儿茶素-3-棓酸盐(EGCG)这种植物化合物在乳腺癌模型中对 PRMT5 和 EZH2 的抑制潜力:方法:结合体内和体外试验,对一系列植物化合物进行了筛选。采用分子对接法评估了 EGCG 与人类 PRMT5:MEP50 和 EZH2 之间的相互作用。通过表面等离子体共振研究验证了结合效率,并通过体外甲基化、Western 印迹、酶联免疫吸附和细胞检测获得了抑制潜力。EGCG 的体内疗效在细胞系衍生的小鼠异种移植模型上进行了验证:结果:EGCG与PRMT5:MEP50复合物和EZH2,尤其是在SAM结合位点上,发生了强有力的相互作用。表面等离子共振分析表明,在纳摩尔浓度下,EGCG与PRMT5-MEP50的结合亲和力比与EZH2的结合亲和力更强。体外试验证实,EGCG 能够抑制 PRMT5 和 EZH2,从而导致它们的催化产物(即 H4R3me2s 和 H3K27me3)减少。EGCG可诱导体内自噬和细胞凋亡。体内研究表明,随着组蛋白抑制标记的减少,肿瘤大小和增殖标记物ki67均显著缩小:结论:研究结果表明,EGCG 能有效抑制 PRMT5 和 EZH2,突出了其在癌症联合治疗策略中的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
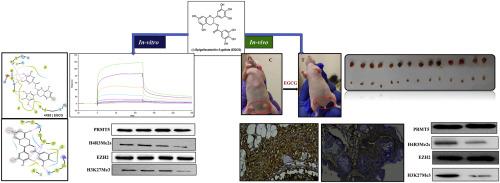
Epigallocatechin-3-gallate inhibit the protein arginine methyltransferase 5 and enhancer of Zeste homolog 2 in breast cancer both in vitro and in vivo
Purpose
Histone methyltransferases are enzymes that selectively methylate lysine or arginine residues on both histone and non-histone proteins, categorized into lysine methyltransferases and arginine methyltransferases. Notably, EZH2 and PRMT5 are known for catalyzing trimethylation of H3 at K27 and symmetric dimethylation of H4 at R3, respectively. These methylation events are recognized as characteristic histone-repressive marks in cancer. The over expression of PRMT5 and EZH2 were reported in various cancers and recognized as a drug target. The study aims to explore the inhibitory potential of phytocompound, Epigallocatechin-3-gallate (EGCG), against PRMT5 and EZH2 in the breast cancer model.
Methods
Screening of an array of phytocompounds was conducted through a combination of in-silico and in-vitro assays. Interactions between EGCG and human PRMT5: MEP50 and EZH2 were evaluated using molecular docking. Binding efficiency was validated, by Surface Plasmon Resonance studies and inhibitory potential was accessed by in vitro methylation followed by western blots, ELISA, and cell-based assays. In-vivo efficacy of EGCG was carried on cell line derived mice xenograft model.
Results
EGCG demonstrated robust interactions with PRMT5:MEP50 complex and EZH2, particularly within the SAM binding site. Surface Plasmon Resonance analysis revealed strong binding affinity in nanomolar concentrations, particularly with PRMT5-MEP50 compared to EZH2. In-vitro assays confirmed EGCG's ability to inhibit PRMT5 and EZH2, leading to a decrease in their catalytic products, namely H4R3me2s and H3K27me3, respectively. EGCG treatment induced both autophagy and apoptosis invitro. In-vivo studies demonstrated significant reductions in tumor size and the proliferation marker ki67, accompanied by a decrease in histone repressive marks.
Conclusion
The findings suggest that EGCG effectively inhibits PRMT5 and EZH2, underscoring its potential for combined therapeutic strategies in cancer treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


