从海洋甲壳类动物贝壳中分离出的 Aneurinibacillus aneurinilyticus 细胞外几丁质脱乙酰化酶的表征
IF 4.8
Q1 MICROBIOLOGY
引用次数: 0
摘要
壳聚糖是一种具有广泛应用前景的生物聚合物。它是甲壳素的脱乙酰产物。商业上,壳聚糖是通过苛刻的热化学工艺从甲壳素中生产出来的,这种工艺存在一些缺点,而且脱乙酰产物不均匀。甲壳素可通过使用甲壳素脱乙酰酶(CDA)进行酶脱乙酰转化为壳聚糖,从而生产出具有特定脱乙酰度的壳聚糖。CDA 主要从真菌中提取,其次是细菌和昆虫。从真菌中提取 CDA 的过程较为复杂,对人体健康有一定风险,包括皮肤损伤。因此,筛选有效的细菌 CDA 是当务之急。在这项研究中,我们首次从海洋蟹壳的漂洗水中分离出了一株 Aneurinibacillus aneurinilyticus 细菌,并发现它是一种强效的 CDA 生产者。对 A. aneurinilyticus 的细胞外 CDA 进行了部分纯化,发现该酶的特异性活性为 569.73 U/mg 蛋白。纯化样品的 SDS-PAGE 分析显示 CDA 有两种异构体,分子量分别为 27 kD 和 45 kD。纯化的 CDA 的最适 pH 值和温度分别为 7.4 和 37 °C。对于对硝基乙酰苯胺等非几丁质底物,部分纯化酶的 Km 值和 Vmax 值分别为 98.455 µM 和 909.09 µmole/min。对于乙二醇甲壳素、N-乙酰葡糖胺六聚物和胶体甲壳素等几丁质底物,该酶的 Km 值分别为 96.96、111.75 和 127.86 µM,Vmax 值分别为 23.31、10.12 和 10.772 µmole/min。锰和镁等金属离子大大提高了 CDA 的产量和活性,而镉和钴则强烈抑制了其活性。这项研究的发现进一步证实了这种酶遵循 Michaelis-Menten 方程,具有工业应用潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
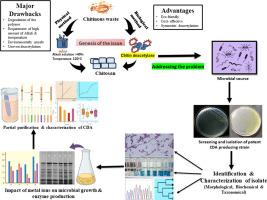
Characterization of extracellular chitin deacetylase from Aneurinibacillus aneurinilyticus isolated from marine crustacean shell
Chitosan is a promising biopolymer with wide range of applications. It is the deacetylated product of chitin. Commercially, it is produced from chitin via a harsh thermochemical process that has several shortcomings and heterogenous deacetylation product. Chitin can be transformed into chitosan through enzymatic deacetylation using chitin deacetylase (CDA), enabling the production of chitosan with a specific degree of deacetylation. CDA is primarily extracted from fungi followed by bacteria and insects. The extraction of CDA from fungus is more complex, possess several health risks for human including skin lesions. Therefore, screening of potent bacterial CDA is the need of the hour. In this study, for the first time we have isolated a bacterial strain Aneurinibacillus aneurinilyticus from the rinsed water of marine crab shell, and it was found to be a potent CDA producer. The extracellular CDA from A. aneurinilyticus has been partially purified and the specific activity of the enzyme was found to be 569.73 U/ mg protein. SDS-PAGE profiling of the purified sample depicts two isomers of CDA with molecular weights of 27 kD and 45 kD. The pH and temperature optima of the purified CDA were found to be 7.4 and 37 °C, respectively. The partially purified enzyme has Km and Vmax values of 98.455 µM and 909.09 µmole/min, for non-chitinous substrate such as p-nitroacetanilide. For chitinous substrates like glycol chitin, N-acetyl glucosamine hexamer and colloidal chitin, the enzyme exhibited Km of 96.96, 111.75 and 127.86 µM, respectively, Vmax for these substrates were 23.31, 10.12 and 10.772 µmole/min, respectively. Metal ions like Mn and Mg considerably boost the production and activity of CDA, whereas Cd and Co strongly inhibit its activity. Insights from this study further substantiate that this enzyme follows Michaelis-Menten equation and has potential for industrial applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Current Research in Microbial Sciences
Immunology and Microbiology-Immunology and Microbiology (miscellaneous)
CiteScore
7.90
自引率
0.00%
发文量
81
审稿时长
66 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


