优化α-磷酸三钙骨水泥复合配方:生物活性玻璃粒度的关键作用
IF 7.9
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
磷酸钙水门汀(CPCs)因其固有的骨诱导特性而被广泛用作骨移植材料,但它们往往缺乏足够的生物性能,无法在缺损部位实现有效的骨愈合。在 CPC 中加入介孔生物活性玻璃 (MBG) 可以提高孔隙率、促进降解并增加可用表面积,从而提供一种解决方案。在这项研究中,我们将 MBG 纳入 CPC,并评估了不同 MBG 粒径(20 微米、38 微米和 100 微米)对 CPC 的凝固特性、微观结构、机械强度和初步细胞反应的影响。研究表明,20 微米的 MBG 颗粒能显著改善 CPC 的凝固特性和抗压强度,而 38 微米的颗粒则能促进降解和离子释放,有利于磷灰石的形成。研究发现,MBG 的加入可促进微结构的均匀性并促进磷灰石的形成,而颗粒大小会直接影响这些结果。生物相容性评估表明,这种材料没有细胞毒性,细胞反应良好(与对照组相比,细胞存活率为 92%)也证明了这一点。这些发现强调了 MBG 颗粒大小对开发用于生物医学应用的先进 CPC 的关键影响,为未来的设计和优化策略提供了指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
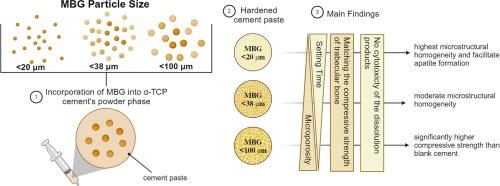
Optimizing α-tricalcium phosphate bone cement composite formulations: The critical role of bioactive glass particle size
Calcium phosphate cements (CPCs) have been extensively utilized as bone grafting material due to their inherent osteoconductive properties, although they often lacked sufficient biological performance for effective bone healing at the defect site. Incorporating mesoporous bioactive glass (MBG) into CPCs offers a solution by improving porosity, promoting degradation and increasing the available surface area. In the scope of this study, we integrated MBG into CPCs and assessed the impact of varying MBG particle sizes (<20 µm, <38 µm, <100 µm) on the setting characteristics, microstructure, mechanical strength, and preliminary cell response of CPCs. Investigations revealed that < 20 µm MBG particles significantly improved the setting characteristics and compressive strength of CPCs, while < 38 µm particles promoted degradation and ion release, facilitating apatite formation. MBG incorporation was found to promote microstructural homogeneity and facilitate apatite formation, with particle size directly affecting these outcomes. Biocompatibility assessments indicated no cytotoxic effects, supported by the favorable cellular responses (> 92 % viability compared to control group). These findings underscore the critical impact of MBG particle size on developing advanced CPCs for biomedical applications, guiding future design and optimization strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Materials & Design
Engineering-Mechanical Engineering
CiteScore
14.30
自引率
7.10%
发文量
1028
审稿时长
85 days
期刊介绍:
Materials and Design is a multi-disciplinary journal that publishes original research reports, review articles, and express communications. The journal focuses on studying the structure and properties of inorganic and organic materials, advancements in synthesis, processing, characterization, and testing, the design of materials and engineering systems, and their applications in technology. It aims to bring together various aspects of materials science, engineering, physics, and chemistry.
The journal explores themes ranging from materials to design and aims to reveal the connections between natural and artificial materials, as well as experiment and modeling. Manuscripts submitted to Materials and Design should contain elements of discovery and surprise, as they often contribute new insights into the architecture and function of matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


