利用乙二胺四乙酸二钾盐直接合成掺杂 N 的多孔碳材料,用于快速吸附水溶液中的六价铬
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
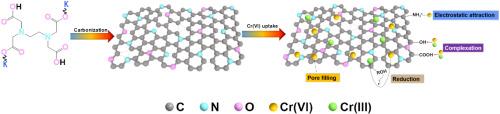
开发用于去除痕量污染物的多功能、可持续和高效的掺 N 多孔碳材料引起了人们的极大兴趣。传统的多孔碳材料制备方法通常需要使用强效且有害的活化剂,如 KOH、H3PO4 和 ZnCl2,这不仅限制了生产设备的选择,还会对环境造成危害。在本研究中,我们介绍了一种自活化方法,用于合成专门用于去除六价铬[Cr(VI)]的掺杂 N 的多孔碳材料。这种方法包括在不同温度下,在不使用任何活化剂的情况下,在 N2 大气中对 EDTA 二钾盐进行直接碳化。系统研究了吸附剂用量、pH 值、温度、六价铬离子的初始浓度以及竞争离子对六价铬吸附行为的影响。研究结果表明,最佳的吸附条件是 pH 值为 2,吸附剂用量为 1.0 g L-1。此外,Cr(VI) 在掺 N 多孔碳上的快速吸附遵循 Freundlich 等温线和伪二阶动力学模型。值得注意的是,吸附剂 KNC-700 的比表面积高达 2124.6 m2 g-1,孔隙结构非常清晰。它在 328 K 时对 Cr(VI) 的最大吸附容量为 270.3 mg g-1,平衡时间为 20 分钟。进一步的机理研究表明,Cr(VI)的有效吸附主要是通过物理化学吸附实现的,其中涉及静电相互作用和还原反应。这项研究为多孔碳材料的制备及其在解决六价铬污染方面的潜在应用提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Direct synthesis of N-doped porous carbon materials from EDTA dipotassium salt for the rapid adsorption of hexavalent chromium from aqueous solutions
The development of versatile, sustainable, and efficient N-doped porous carbon materials for the removal of trace contaminants has attracted considerable interest. Traditional methods for preparing porous carbon materials often involve the use of potent and hazardous activating agents such as KOH, H3PO4, and ZnCl2, which not only restrict the choice of production equipment but also pose environmental hazards. In this study, we introduce a self-activating method to synthesize N-doped porous carbon materials specifically designed for the removal of hexavalent chromium [Cr(VI)]. This approach involves the direct carbonization of EDTA dipotassium salt at different temperatures under a N2 atmosphere without any activators. The influence of adsorbent dosages, pH, temperature, initial concentration of Cr(VI) ions, and competing ions on the adsorption behavior of Cr(VI) was systematically examined. The findings reveal that the optimal conditions for adsorption are a pH of 2 and an adsorbent dosage of 1.0 g L−1. Furthermore, Cr(VI) rapid adsorption onto N-doped porous carbon follows Freundlich isotherm and pseudo-second-order kinetic models. Notably, adsorbent KNC-700 exhibits an exceptional specific surface area of 2124.6 m2 g−1 and a well-defined pore structure. It shows maximum adsorption capacity for Cr(VI) of 270.3 mg g−1 at 328 K with an equilibrium time of 20 min. Further mechanistic investigations suggest that efficient uptake of Cr(VI) primarily occurs through physico chemical adsorption involving electrostatic interactions and reduction reactions. This study provides novel insights into the preparation of porous carbon materials and their potential applications in addressing Cr(VI) pollution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


