新型水热法制造碱土金属和过渡金属硫化物(BaS/CuS)复合材料:超级电容器应用电极
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
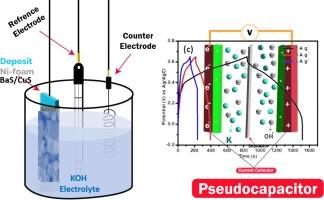
化石燃料的快速利用和开采导致了以污染和能源枯竭为特征的生态系统的建立。尽管如此,加快替代能源生产和能源存储设备制造方法的发展至关重要。在这种情况下,超级电容器(SC)成为性能最佳的储能设备。超级电容器可以通过采用多种电极材料(包括过渡金属氧化物、瑀、过氧化物和尖晶石)来提高其电化学效率。作为改进超级电容器应用的电极,本研究采用水热法制备了一种新型 BaS/CuS 复合材料。物理表征证实了制备的成功。BET 结果表明,制备的 BaS/CuS 复合材料具有更高的表面积(87.4 m2 g-1)。在碱性介质中通过三种电极配置对 BaS/CuS 复合电极进行了测试,结果表明该电极具有高铯(908.7F/g,1 A/g 时)、高能量密度和功率密度(分别为 68.6 Wh kg-1 和 368.6 W kg-1)以及良好的循环稳定性。根据之前的研究结果,合成的电极有望用于超级电容器。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Novel hydrothermally fabricated alkaline earth metal and transition metal sulfide (BaS/CuS) composite: An electrode for supercapacitor application
The speedy utilization and exploitation of fossil fuels lead to establishment of an ecosystem marked by pollution and the depletion of energy resources. Nevertheless, expediting the progress of alternative approaches to generate energy and fabricate energy storage devices is crucial. In such cases, supercapacitors (SC) emerged as the most auspicious energy storage device in terms of performance. Supercapacitors can have their electrochemical efficiency enhanced by employing several electrode materials, including transition metal oxides, chalcogenides, perovskites, and spinel. As an electrode for improved SC applications, a new BaS/CuS composite was created in this work using a hydrothermal method. The physical characterizations confirmed the proof of successful fabrication. BET results show that the prepared composite of the BaS/CuS has enhanced surface area (87.4 m2 g−1). BaS/CuS composite electrode was tested in alkaline media via three electrode configurations, and it demonstrated the high Cs (908.7F/g at 1 A/g), high energy density, and power density of 68.6 Wh kg−1 and 368.6 W kg−1 respectively, and good cyclic stability. According to the previously described findings, the synthesized electrode is promising for supercapacitor use.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


