酶合成的直链淀粉样嵌合异麦芽糖的分子结构及其对磺胺沙拉嗪原药的包裹作用
IF 12.5
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
线性嵌合α-(1→4)-和α-(1→6)-葡糖苷段之间的糖化作用在其整个结构中表现出功能特性。在这项研究中,我们用酶法合成了三个新系列的嵌合型非还原异麦芽糖(N-IMS-n/m),每个系列都具有非还原末端恒定的 n、α-(1→4)-片段(平均聚合度 DP = 22-25)和不同的 m、α-(1→6)-主链长度(DP = 7-53)。合成的化合物-N-IMS-25/7、N-IMS-24/19 和 N-IMS-22/53 与直链淀粉(DP = 28)以及以前的 N-IMS-15/35 和 D-IMS-28.3/13/3 样品进行了比较。D-IMS 指的是非还原端和还原端均具有双 α-(1→4)- 段的糖。采用相溶解度测定法评估了与芳香族原药磺胺沙拉嗪(SZ)的结合亲和力,然后进行冻融。广角 X 射线散射显示了 B 型晶体形态,随着 α-(1→6) 段的增加,结晶度普遍降低。有趣的是,即使在封装 SZ 后,B 型晶体结构仍然保持不变,这与药物封装后向 V 型晶体转变的普遍现象截然不同。多角度动态光散射和小角度 X 射线散射显示,在没有 SZ 和有 SZ 的情况下,溶液态形态都错综复杂。糖化作用有助于保持结构的组织性和完整性,即使在加入大分子 SZ 后也是如此。本文章由计算机程序翻译,如有差异,请以英文原文为准。
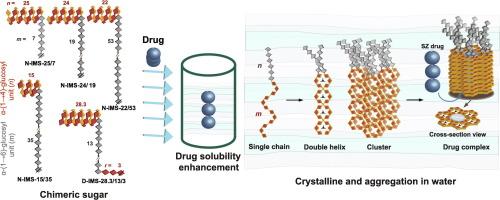
Molecular structure of enzyme-synthesized amylose-like chimeric isomaltomegalosaccharides and their encapsulation of the sulfasalazine prodrug
The glucoconjugation between linear chimeric α-(1→4)- and α-(1→6)-glucosidic segments exhibits functional properties throughout their structure. In this study, we enzymatically synthesized three new series of chimeric nonreducing isomaltomegalosaccharides (N-IMS-n/m), each featuring a constant n, α-(1→4)-segment (average degree of polymerization, DP = 22–25) at the nonreducing terminal, and varying m, α-(1→6)-main chain lengths (DP = 7–53). The synthesized compounds—N-IMS-25/7, N-IMS-24/19, and N-IMS-22/53—were compared to amylose (DP = 28) and previous samples of N-IMS-15/35 and D-IMS-28.3/13/3. D-IMS refers to a sugar with double α-(1→4)-segments at both the nonreducing and reducing ends. The binding affinity to the aromatic prodrug sulfasalazine (SZ) was assessed using a phase-solubility assay, followed by freeze-thawing. Wide-angle X-ray scattering revealed B-type crystalline patterns in bulk, and the crystallinity generally reduced with the increasing α-(1→6) segment. Interestingly, the B-type crystal structure was maintained even after SZ encapsulation, in contrast to the more common transition to V-type crystals upon drug encapsulation. Multi-angle dynamic light scattering and small-angle X-ray scattering revealed an intricate solution-state morphology, both in the absence and presence of SZ. Glucoconjugation aids in maintaining structural organization and integrity, even after the incorporation of the large SZ molecule.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbohydrate Polymers
化学-高分子科学
CiteScore
22.40
自引率
8.00%
发文量
1286
审稿时长
47 days
期刊介绍:
Carbohydrate Polymers stands as a prominent journal in the glycoscience field, dedicated to exploring and harnessing the potential of polysaccharides with applications spanning bioenergy, bioplastics, biomaterials, biorefining, chemistry, drug delivery, food, health, nanotechnology, packaging, paper, pharmaceuticals, medicine, oil recovery, textiles, tissue engineering, wood, and various aspects of glycoscience.
The journal emphasizes the central role of well-characterized carbohydrate polymers, highlighting their significance as the primary focus rather than a peripheral topic. Each paper must prominently feature at least one named carbohydrate polymer, evident in both citation and title, with a commitment to innovative research that advances scientific knowledge.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


