用量子点碳装饰提高氧化锌光电极的电荷转移和光电化学活性
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-11-13
DOI:10.1016/j.jphotochem.2024.116141
引用次数: 0
摘要
氧化锌(ZnO)是一种前景广阔的半导体,在光催化、电子学和光电子学等各个领域都具有巨大潜力。在光电化学(PEC)设备领域,氧化锌光电极已成为利用太阳能驱动氢气和氧气生产的关键元件。然而,氧化锌固有的宽带隙带来了挑战,它阻碍了光吸收率,并助长了快速的电子-空穴重组。为了解决这个问题,人们巧妙地在氧化锌中加入了通过水热法合成的碳量子点(CQDs)和掺氮碳量子点(N-CQDs)。值得注意的是,在 1 M NaOH 碱性溶液中制造的氧化锌光电极呈现纳米立方体结构,而在 1 M 尿素溶液中制造的氧化锌光电极则采用纳米棒构型。其中,ZnO/尿素具有增强光电流的特点,已被选中与浓度均为 8.5 毫克/毫升的 CQDs 和 N-CQDs 一起用于进一步增强。紫外可见光谱分析显示,与原始 ZnO/Urea 相比,ZnO/Urea/CQDs 和 ZnO/Urea/NCQDs 的光吸收能力更强,同时量子带隙分别降低到 2.89 eV 和 3.01 eV。值得注意的是,与 RHE 相比,ZnO/Urea/CQDs 在 1.23 V 时的光电流密度达到了 0.56 mA/cm2。这项研究强调了量子点装饰在提高 ZnO 光电极电荷转移速率和增强光催化活性方面的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
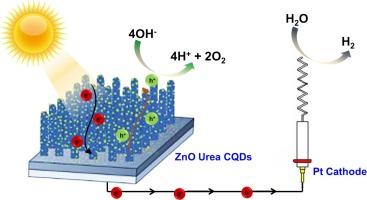
Boosting charge transfer and photoelectrochemical activity in ZnO photoelectrodes with quantum dot carbon decorations
Zinc oxide (ZnO) stands as a promising semiconductor with vast potential across various domains, including photocatalysis, electronics, and optoelectronics. In the realm of photoelectrochemical (PEC) devices, ZnO photoelectrodes have emerged as pivotal components for harnessing solar energy to drive hydrogen and oxygen production. However, the inherent wide band gap of ZnO poses challenges, retarding light absorption rates and fostering rapid electron-hole recombination. To tackle this, ZnO has been ingeniously augmented with carbon quantum dots (CQDs) and nitrogen-doped carbon quantum dots (N-CQDs), synthesized via the hydrothermal method. Notably, ZnO photoelectrodes fabricated in 1 M NaOH alkaline solution exhibit a nanocubic structure, while those produced in 1 M urea solution adopt a nanorod configuration. Among these, ZnO/Urea, characterized by enhanced photocurrent, has been selected for further enhancement with CQDs and N-CQDs, both at a concentration of 8.5 mg/ml. UV–Vis spectra analysis reveals that ZnO/Urea/CQDs and ZnO/Urea/NCQDs exhibit superior light absorption compared to pristine ZnO/Urea, concurrently reducing the quantum band gap to 2.89 eV and 3.01 eV, respectively. Notably, ZnO/Urea/CQDs achieve a noteworthy photocurrent density of 0.56 mA/cm2 at 1.23 V vs. RHE. This study underscores the pivotal role of quantum dot decoration in enhancing charge transfer rates and augmenting photocatalytic activity within ZnO photoelectrodes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


