氯雷他定作为 X65 钢在 1M HCl 水溶液中的缓蚀剂的电化学和理论评估
IF 1.3
4区 化学
Q4 ELECTROCHEMISTRY
International Journal of Electrochemical Science
Pub Date : 2024-11-02
DOI:10.1016/j.ijoes.2024.100843
引用次数: 0
摘要
目前,主要由碳钢合金制造的石油工业基础设施在清洗和维护过程中由于使用酸性溶液而暴露在腐蚀性环境中,因此寻找环保、经济和高效的保护替代品是腐蚀科学研究的一个活跃领域。在这项工作中,通过电化学阻抗光谱(EIS)和电位极化(PDP)研究了氯雷他定(C22H23ClN2O2)在室温酸性介质中作为 X65 钢缓蚀剂的用途。使用扫描电子显微镜(SEM)和能量色散光谱(EDS)进行了表面分析。理论计算(密度泛函理论,DFT)用于阐明氯雷他定与铁簇模型之间的相互作用。根据 PDP 和 EIS 分析,抑制剂效率分别达到 93.69% 和 85.21% 的最大值。热力学分析表明,吸附自由能(ΔGads=-39.41 kJ/mol)与混合吸附过程相对应。此外,七元环、吡啶和氯苯基团被确定为优先活性位点。此外,从金属原子到有机分子的静电相互作用也解释了物理相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
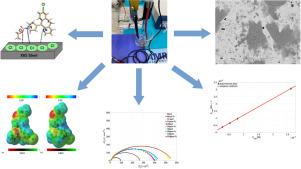
Electrochemical and theoretical evaluation of loratadine as corrosion inhibitor for X65 steel in 1M HCl aqueous solution
Currently oil industry infrastructure, mainly fabricated in carbon steel alloys, is exposed to aggressive environments due to the use of acid solutions during cleaning and maintenance operations, the search for eco friendly, economic and efficient protection alternatives is an active field of research in corrosion science. In this work the use of loratadine () as corrosion inhibitor for X65 steel in acidic media at room temperature is studied by means of electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization (PDP). Surface analysis was carried out using scanning electronic microscopy (SEM) and energy dispersive spectroscopy (EDS). Theoretical calculations (density functional theory, DFT) were used to elucidate the interaction between loratadine and an iron cluster model. Inhibitor efficiency increases with concentration reaching a maximum value of 93.69% according to PDP and 85.21% according to EIS. Thermodynamic analysis revealed that free energy of adsorption ( kJ/mol) corresponds to a mixed adsorption process. Furthermore, the seven membered ring, pyridine and chlorophenyl groups were determined as the preferential active sites. Additionally, electrostatic interactions due to the transference from metal atoms to the organic molecules explains the physical interaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.00
自引率
20.00%
发文量
714
审稿时长
2.6 months
期刊介绍:
International Journal of Electrochemical Science is a peer-reviewed, open access journal that publishes original research articles, short communications as well as review articles in all areas of electrochemistry: Scope - Theoretical and Computational Electrochemistry - Processes on Electrodes - Electroanalytical Chemistry and Sensor Science - Corrosion - Electrochemical Energy Conversion and Storage - Electrochemical Engineering - Coatings - Electrochemical Synthesis - Bioelectrochemistry - Molecular Electrochemistry

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


