氢氧离子诱导聚四氟乙烯-共六氟丙烯共聚物(FEP)在亚临界水中完全矿化
IF 5.8
2区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
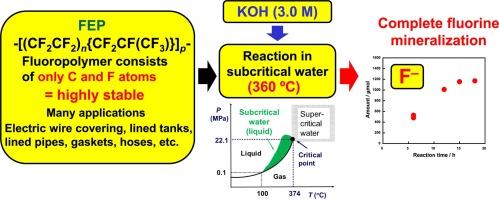
研究了聚四氟乙烯-六氟丙烯共聚物(FEP)在超临界和亚临界水中的分解情况,目的是进行废物处理。通过使用氧化剂(O2、H2O2)或碱性试剂(KOH)对这种分解的效率进行了检验。在这些条件下,气相中检测到了微量的 CHF3,其含量随着反应时间的延长而减少。H2O2 的反应活性略高于 O2。与此相反,与 KOH 的反应可诱导有效的氟矿化。当 FEP 在 360 °C 的 3.0 M KOH 溶液中反应 18 小时后,氟产量达到 98%。因此,实现了完全的氟矿化,在气相中只生成了极少量的 CO2(产率为 ∼ 0%),没有生成 CHF3。此外,19F NMR 光谱和燃烧离子色谱法显示,反应溶液在反应过程中不含有任何有机氟化合物。FEP 分解后将 F- 释放到反应溶液中,在残留物中形成无定形碳。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hydroxide-ion induced complete mineralization of poly(tetrafluoroethylene-co-hexafluoropropylene) copolymer (FEP) in subcritical water
Decomposition of poly(tetrafluoroethylene-co-hexafluoropropylene) copolymer (FEP) in supercritical and subcritical water was investigated with the aim of waste treatment. The efficiency of such decomposition was examined by using either oxidizing agents (O2, H2O2) or an alkaline reagent (KOH). When O2 was chosen, the highest F– and CO2 yields, 75 % and 64 %, respectively, were obtained from a reaction of FEP in supercritical water at 384 °C for 24 h. Under these conditions, traces of CHF3 were detected in the gas phase, the amount of which decreased with increasing the reaction time. H2O2 gave slightly higher reactivity than O2. In contrast, reactions with KOH induced efficient fluorine mineralization. When FEP reacted in 3.0 M KOH solution at 360 °C for 18 h, the F– yield reached 98 %. Hence, complete fluorine mineralization was achieved, where very little CO2 (∼0% yield) and no CHF3 were generated in the gas phase. Furthermore,19F NMR spectroscopy and combustion-ion chromatography revealed that the reaction solutions did not contain any organofluorine compounds during the reactions. FEP decomposed, releasing F– into the reaction solution, resulting in the formation of amorphous carbon in the residue.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

European Polymer Journal
化学-高分子科学
CiteScore
9.90
自引率
10.00%
发文量
691
审稿时长
23 days
期刊介绍:
European Polymer Journal is dedicated to publishing work on fundamental and applied polymer chemistry and macromolecular materials. The journal covers all aspects of polymer synthesis, including polymerization mechanisms and chemical functional transformations, with a focus on novel polymers and the relationships between molecular structure and polymer properties. In addition, we welcome submissions on bio-based or renewable polymers, stimuli-responsive systems and polymer bio-hybrids. European Polymer Journal also publishes research on the biomedical application of polymers, including drug delivery and regenerative medicine. The main scope is covered but not limited to the following core research areas:
Polymer synthesis and functionalization
• Novel synthetic routes for polymerization, functional modification, controlled/living polymerization and precision polymers.
Stimuli-responsive polymers
• Including shape memory and self-healing polymers.
Supramolecular polymers and self-assembly
• Molecular recognition and higher order polymer structures.
Renewable and sustainable polymers
• Bio-based, biodegradable and anti-microbial polymers and polymeric bio-nanocomposites.
Polymers at interfaces and surfaces
• Chemistry and engineering of surfaces with biological relevance, including patterning, antifouling polymers and polymers for membrane applications.
Biomedical applications and nanomedicine
• Polymers for regenerative medicine, drug delivery molecular release and gene therapy
The scope of European Polymer Journal no longer includes Polymer Physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


