聚丙烯腈/聚偏氟乙烯-六氟丙烯的双层不对称界面改性,用于全固态锂金属电池的稳定循环
IF 4.3
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
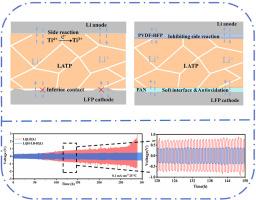
Li1.5Al0.5Ti1.5(PO4)3 (LATP) 固体电解质具有良好的化学稳定性、显著的离子导电性和低成本,因此被广泛研究。然而,高界面阻抗和与电极的可怕副反应限制了 LATP 在锂金属电池中的应用。为解决这一问题,研究人员在 LATP 的两侧引入了聚偏二氟乙烯-六氟丙烯(PVDF-HFP)和聚丙烯腈(PAN)层,制备出 PVDF-HFP@LATP-Bi2O3@PAN (H-LB-P),以满足不同电极与 LATP 之间的界面要求。具有 Li+ 导电性和粘附性的 PAN 改性层实现了 LATP 与阴极之间的紧密连接和完美的界面接触。同时,PVDF-HFP 层有效隔离了锂金属阳极(锂)与 LATP 之间的副反应。此外,X 射线光电子能谱(XPS)表明,PVDF-HFP 与锂金属反应形成了稳定的锂金属界面层。因此,Li||H-B-H||Li 电池在 0.1 mA cm-2 下可稳定循环 300 小时。在 25 °C、0.1C 条件下循环 100 次后,Li||H-LB-P||LiFePO4 电池的初始放电容量为 153.06 mAh g-1,保持率为 82%。这项工作为氧化物电解质在锂金属电池中的应用提供了一种可行的设计。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Double-layer asymmetric interfacial modification of polyacrylonitrile/poly(vinylidene fluoride-co-hexafluoropropylene) for stable cycling of all-solid-state lithium metal batteries
The Li1.5Al0.5Ti1.5(PO4)3 (LATP) solid electrolyte is widely investigated owing to good chemical stability, remarkable ionic conductivity and low cost. However, the high interfacial impedance and terrible side reaction with electrode limits the application of LATP in lithium metal batteries. To address this issue, the poly (vinylidene fluoride-co-hexafluoropropylene (PVDF-HFP) and polyacrylonitrile (PAN) layers were introduced to both sides of LATP to prepare PVDF-HFP@LATP-Bi2O3@PAN (H-LB-P) for meeting the interfacial requirements between different electrodes and LATP. The PAN modified layer with Li+ conductivity and adhesion achieved to tight connection and perfect interfacial contact between LATP and cathode. Meanwhile, the PVDF-HFP layer effectively isolates the side reaction between lithium metal anode (Li) and LATP. Moreover, the X-ray photoelectron spectroscopy (XPS) indicates that PVDF-HFP reacts with Li-metal to form a stable LiF interfacial layer. As a result, the Li||H–B–H||Li cell exhibits stable cycling over 300 h at 0.1 mA cm−2. The Li||H-LB-P||LiFePO4 battery delivers the favorable initial discharge capacity of 153.06 mAh g−1 and retention rate (82 %) after 100 cycles at 0.1C for 25 °C. This work provides a viable design for application of oxide electrolyte in lithium metal batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


