小檗胺可预防 SARS-CoV-2 的进入和传播
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
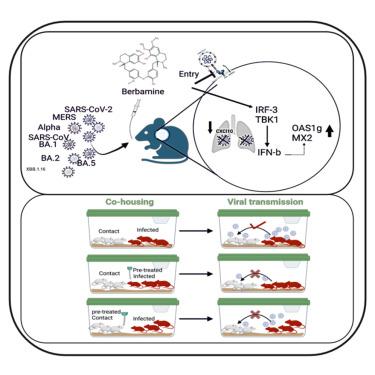
有效的抗病毒药物对于抗击 COVID-19 和未来的流行病至关重要。尽管许多化合物在体外显示出抗病毒活性,但只有少数化合物在体内对 SARS-CoV-2 保持有效。在这里,我们发现小檗胺(Berb)对 SARS-CoV、MER-CoV、SARS-CoV-2 及其变种(包括 XBB.1.16 变种)有效。在 hACE2.Tg 小鼠中,Berb 通过两种不同的机制抑制 SARS-CoV-2 的复制:抑制尖峰介导的病毒进入和增强感染期间的抗病毒基因表达。将 Berb 与雷米地韦(RDV)、氯法齐明(Clof)和方胆啉(Fcn)联合使用,几乎可以消除病毒载量,促进急性 SARS-CoV-2 感染及其变种的恢复。与预处理或未处理的感染小鼠直接接触的同舍小鼠的病毒载量可忽略不计,肺部病理变化减轻,病毒脱落减少,这表明 Berb 可有效阻止病毒传播。这种广谱活性使 Berb 成为一种很有前景的预防或治疗 betacoronaviruses 的选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Berbamine prevents SARS-CoV-2 entry and transmission
Effective antiviral drugs are essential to combat COVID-19 and future pandemics. Although many compounds show antiviral in vitro activity, only a few retain effectiveness in vivo against SARS-CoV-2. Here, we show that berbamine (Berb) is effective against SARS-CoV, MER-CoV, SARS-CoV-2 and its variants, including the XBB.1.16 variant. In hACE2.Tg mice, Berb suppresses SARS-CoV-2 replication through two distinct mechanisms: inhibiting spike-mediated viral entry and enhancing antiviral gene expression during infection. The administration of Berb, in combination with remdesivir (RDV), clofazimine (Clof) and fangchinoline (Fcn), nearly eliminated viral load and promoted recovery from acute SARS-CoV-2 infection and its variants. Co-housed mice in direct contact with either pre-treated or untreated infected mice exhibited negligible viral loads, reduced lung pathology, and decreased viral shedding, suggesting that Berb may effectively hinder virus transmission. This broad-spectrum activity positions Berb as a promising preventive or therapeutic option against betacoronaviruses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


