在阴离子主机中选择性识别水阴离子
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
利用功能化三叉配体、FeII 盐和水溶性磺化甲酰基吡啶成分,可以通过多组分自组装工艺合成水溶性 Fe4L44- 笼。笼状配体可溶于纯水溶液,总体电荷为 4-,尽管具有阴离子性质,但仍能在宿主空腔中结合适当大小的非配位阴离子。PF6- 或 AsF6- 等阴离子会占据内腔,而太小(BF4-)或太大(NTf2-)的阴离子则不会被包裹。外部的阴离子电荷和立体阻塞的配体核心限制了结合阴离子的交换率,因为充满阴离子的笼子在数周内不会发生交换,而空笼子对添加的 PF6- 的内化需要数周时间,尽管空腔对 PF6- 离子有很强的亲和力。未来,这种识别机制可用于控制阴离子的释放,以满足环境应用的需要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
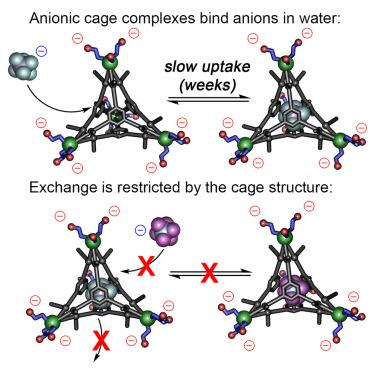
Selective aqueous anion recognition in an anionic host
Water-soluble Fe4L44− cages can be synthesized in a multicomponent self-assembly process exploiting functionalized trigonal ligands, FeII salts, and water-soluble sulfonated formylpyridine components. The cages are soluble in purely aqueous solution and display an overall 4− charge, but are capable of binding suitably sized non-coordinating anions in the host cavity despite their anionic nature. Anions such as PF6− or AsF6− occupy the internal cavity, whereas anions that are too small (BF4−) or too large (NTf2−) are not encapsulated. The external anionic charge and sterically blocked ligand cores limit the exchange rate of bound anions, as no exchange is seen over a period of weeks with the anion-filled cages, and internalization of added PF6− by an empty cage takes multiple weeks, despite the strong affinity of the cavity for PF6− ions. In the future, this recognition mechanism could be used to control release of anions for environmental applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


