通过在纳米反应器内结合约束效应和路易斯酸碱反应实现类芬顿反应
IF 6.3
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
在类似芬顿的反应中,污染物的降解性能与过一硫酸盐(PMS)的活化活性密切相关。作为一种路易斯碱,路易斯酸碱反应或局部高浓度都能加速 PMS 的活化。为了同时实现这两个目标,本文制备了一种纳米反应器,将 SO42 修饰的 Co3O4 纳米粒子封装在 Co2SiO4 外壳中。借助 SO42- 强大的电子吸引力,Co3+/Co2+ 催化位点上的路易斯酸性得到改善,从而导致快速的路易斯酸碱反应。在封闭效应的作用下,空腔内的 PMS 和生成的活性氧浓度都得到了提高,导致 SO4--+-OH 快速转化为 1O2。得益于这两大优势,纳米反应器在16.0 min时的甲硝唑降解效率为92.2%,矿化效率为68.9%,PMS活化效率为44.0%,PMS利用效率为67.2%,远高于参考催化剂。同时,浸出钴离子浓度仅为 0.48 mg/L,低于无 Co2SiO4 外壳保护的样品。这项研究提供了一种在纳米反应器内设计催化剂表面性质的新方法,并通过限制效应和路易斯酸碱反应的结合实现了类似芬顿反应的加速。本文章由计算机程序翻译,如有差异,请以英文原文为准。
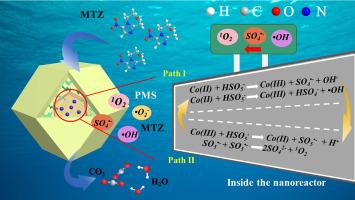
Fenton-like reaction via combining confinement effect and Lewis acid-base reaction inside a nanoreactor
In Fenton-like reaction, the pollutant degradation performance was closely related to the peroxymonosulfate (PMS) activation activity. As a Lewis base, PMS activation could be accelerated by either Lewis acid-base reaction, or the high local concentrations. To simultaneously realize two aims, herein, a nanoreactor was prepared, where SO42− modified Co3O4 nanoparticles were encapsulated inside Co2SiO4 shell. The Lewis acidity on Co3+/Co2+ catalytic sites was improved with the help of SO42− possessed powerful electron attraction, leading to a rapid Lewis acid-base reaction. Under the confinement effect, both PMS and generated reactive oxygen specie concentrations were boosted inside the cavity, resulting in a rapid transformation of SO4•‐+•OH to 1O2. Benefiting from two advantages, metronidazole degradation efficiency over nanoreactor at 16.0 min was 92.2%, mineralization efficiency was 68.9%, PMS activation efficiency was 44.0%, and PMS utilization efficiency was 67.2%, much higher than the reference catalysts. Meanwhile, the leached cobalt ion concentration was only 0.48 mg/L, lower than samples without Co2SiO4 shell protection. This work provided a novel way to engineer catalyst surface property inside a nanoreactor, and realized Fenton-like reaction acceleration via combination of confinement effect and Lewis acid-base reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


