利用熊果(Arctostaphylos uva-ursi. L.)碳化工艺生产生物炭和改性生物炭及其特性:从水溶液中去除 Pb2+、Cd2+ 和罗丹明 B 的吸附容量和动力学研究
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
摘要
在这项研究中,熊果(Arctostaphylos uva-ursi L.)树叶和树枝被用作新型生物质源,通过一步碳化法(800 °C)生产生物炭和改性生物炭(利用二氧化碳和 H3PO4,通过物理和化学活化制造微孔和介孔炭),这些生物炭具有优异的物理化学特性,可有效去除水溶液中的 Pb2+、Cd2+ 离子和合成染料(罗丹明 B - RhB)。结果表明,碳化碳(BL-C)和物理活性碳(BL-CO2)作为微孔吸附剂(比表面积分别为 219.0 m2/g 和 305.5 m2/g),对 Pb2+ 的去除率非常高(BL-C 和 BL-CO2 的去除率分别为 99.8 % 和 99.9 %),而这些吸附剂对 Cd2+ 的去除亲和力适中(分别为 53.5 % 和 48.5 %)。BL-H3PO4 作为介孔吸附剂,具有较低的比表面和较大的孔隙(90.2 m2/g,Dmax = 33.6 nm),对 PhB 的去除率非常高(约 87%)。研究发现,BL-H3PO4 在去除 RhB 的过程中会发生物理吸附,其主要机制是薄膜扩散,边界层效应减弱。吸附过程通过π-π、氢键和静电相互作用进行。Pb2+ 和 Cd2+ 在 BL-CO2 和 BL-C 上的吸附过程是通过物理吸附和化学吸附进行的,但吸附机理不同,分别是扩散和化学吸附相结合(边界层效应增强)和粒子内扩散(边界层效应大大减弱)。本研究发现的一个非常有趣的事实是,金属氧化物表面(如活性炭中的 Cu2O、SiO2 和 ZnO)对镉具有高效的结合力,从而为非金属石墨烯特征提供了物理吸附能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
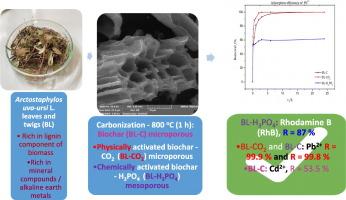
Production and characterization of biochar and modified biochars by carbonization process of bearberry (Arctostaphylos uva-ursi. L.): Adsorption capacities and kinetic studies of Pb2+, Cd2+ and rhodamine B removal from aqueous solutions
In this work, Bearberry (Arctostaphylos uva-ursi L.) leaves and twigs were used as novel biomass source for production of biochar and modified biochars (manufacturing of microporous and mesoporous carbons by physical and chemical activations, using CO2 and H3PO4) via one-step carbonization (800 °C) with excellent physicochemical properties, for effective removal of Pb2+ and Cd2+ ions, and synthetic dye (Rhodamine B - RhB) from aqueous solutions. Results showed that carbonized (BL-C) and physically activated carbons (BL-CO2) as microporous adsorbents (specific surface areas 219.0 m2/g and 305.5 m2/g) show remarkable removal efficiency of Pb2+ (99.8 % and 99.9 %, for BL-C and BL-CO2), while these adsorbents showed moderate affinity for Cd2+ elimination (53.5 % and 48.5 %). BL-H3PO4 as mesoporous adsorbent with lower specific surface and larger pores (90.2 m2/g with Dmax = 33.6 nm), shows very good removal efficiency of PhB (~ 87 %). It was found that physical adsorption occurs during RhB removal onto BL-H3PO4, where dominant mechanism represents film diffusion, with reduced boundary layer effect. Adsorption process takes place over π–π, hydrogen bonding and electrostatic interactions. Adsorption processes of Pb2+ and Cd2+ onto BL-CO2 and BL-C take place via physical and chemical adsorption, but with different type of mechanism, including combination of diffusion and chemisorption (increased effect of boundary layer) and intra-particle diffusion (greatly reduced boundary layer effect), respectively. A very interesting fact found in this study, is that metal oxide surfaces (as Cu2O, SiO2, ZnO present in activated carbons) exhibit an efficient binding towards cadmium, providing physisorption capability onto non-metallic graphene features.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


