简便方法制备的石墨烯量子点的巨大储能能力
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
摘要
超级电池技术的重点是同时具有高能量密度和功率密度的高比容量。本研究比较了石墨烯量子点(GQDs)和氧化石墨烯(GO)的电化学性能。石墨烯量子点(GQDs)是用改进的汉默法合成的。通过简便的化学切割法合成了小于 5 纳米的 GQDs。拉曼分析揭示了 Sp-sp2 键碳在 2198 cm-1 处的高频振动模式。利用基于平面波自洽场(PWSCF)的量子 Espresso 代码计算了不同尺寸 GQDs 的能隙。最高占位分子轨道(HOMO)和最低未占位分子轨道(LUMO)之间的禁止能隙随 GQDs 簇的大小而变化。电化学结果表明,GQDs 具有伪电容器特性,在电流密度为 4 A/g 时,比容量和能量密度分别高达 1516.7C/g 和 425.9 Whkg-1。这与 GO 的双电层电容器 (EDLC) 行为(115C/g)相比非常高。这些结果表明,该材料的电化学性能、导电性和离子传输速率都得到了提高,这意味着该材料可以促进器件内的离子运动,从而实现快速充放电。本文章由计算机程序翻译,如有差异,请以英文原文为准。
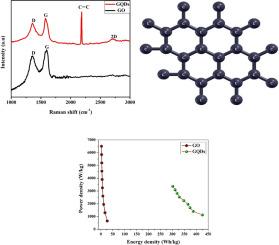
Giant energy storage capacity of graphene quantum dots prepared by facile method
Supercapattery technology focuses on high specific capacity with both high energy density and power density. In the present work, the electrochemical performance of Graphene quantum dots (GQDs) is compared with graphene oxide (GO). GO is synthesized by the modified Hummer's method. Less than 5 nm size of GQDs are synthesized by a facile chemical cutting method. High frequency vibrational mode of sp-sp2 bonded carbon at 2198 cm−1 is revealed from Raman analysis. The energy gap of GQDs of different sizes was calculated using the Quantum Espresso code based on Plane-Wave Self-Consistent Field (PWSCF). The forbidden gap between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) levels with the size of the GQDs cluster. The electrochemical results show that GQDs exhibit pseudocapacitor behavior with high specific capacity and energy density of 1516.7C/g and 425.9 Whkg−1 respectively at current density of 4 A/g. This is very high compared to the electric double layer capacitor (EDLC) behavior (115C/g) of GO. These results illustrate enhanced electrochemical performance, electrical conductivity and ion transport rate implying that the material can facilitate the movement of ions within the device, allowing for fast charging and discharging.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


