热分解法合成的三维 CuO 纳米花和 g-C3N4/CuO/PAN 复合材料的电化学性能
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
摘要
通过热分解合成了 g-C3N4、CuO 和 g-C3N4/CuO/PAN 复合材料,然后进行了全面的表征。利用 X 射线衍射 (XRD)、傅立叶变换红外光谱 (FTIR)、透射电子显微镜 (TEM)、能量色散 X 射线光谱 (EDS) 和场发射扫描电子显微镜 (FESEM) 分析了结构和形态细节。在 g-C3N4、CuO 和 g-C3N4/CuO/PAN 复合材料上进行了电化学实验,包括循环伏安法 (CV)、电化学阻抗光谱法 (EIS) 和电静态充放电法 (GCD)。g-C3N4、CuO 和 PAN 复合材料的稳定性、寿命、电容和电荷存储能力都有显著提高。与单独的 g-C3N4 和 CuO 相比,g-C3N4/CuO/PAN 复合材料表现出更优越的电化学特性。具体来说,在 0.5 M H2SO4 中,电流密度为 1 A/g 时,g-C3N4/CuO/PAN 复合材料的电容为 389 F/g,电容保持稳定性为 99.2%(6000 次循环),优于 CuO(100 F/g)和 g-C3N4(300 F/g)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
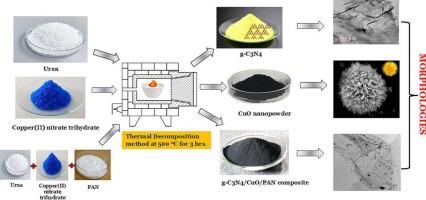
Electrochemical performance of 3D CuO nanoflowers and g-C3N4/CuO/PAN composite synthesized by thermal decomposition method
The synthesis of g-C3N4, CuO, and g-C3N4/CuO/PAN composite was achieved through thermal decomposition, followed by comprehensive characterization. X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDS), and field-emission scanning electron microscopy (FESEM) were utilized to analyze structural and morphological details. The formation of 3D CuO nanoflowers occurred at a temperature of 500 °C.
Electrochemical experiments, including cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and galvanostatic charge-discharge (GCD), were conducted on the g-C3N4, CuO, and g-C3N4/CuO/PAN composite. Incorporating g-C3N4, CuO, and PAN has significantly improved stability, lifespan, capacitance, and charge storage capabilities. The g-C3N4/CuO/PAN composite demonstrated superior electrochemical characteristics compared to g-C3N4 and CuO individually. Specifically, the g-C3N4/CuO/PAN composite exhibited a capacitance of 389 F/g at a current density of 1 A/g in 0.5 M H2SO4 with capacitance retention of 99.2 % stability (6000 cycles), outperforming CuO (100 F/g) and g-C3N4 (300 F/g). g-C3N4/CuO/PAN composite has the potential to revolutionize high-energy storage supercapacitors, inspiring a new wave of innovation in the field of energy storage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


