利用熔盐电解质工艺生产二氧化碳转化碳纳米管,用于钠离子电池的环保型可持续阳极
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
摘要
利用熔盐电化学工艺,从工业二氧化碳排放中合成了二氧化碳转化碳纳米管(CNT),用于钠离子电池(SIB)的生态友好型可持续阳极。合成的 CNT 直径介于 10 纳米到 100 纳米之间,与硬碳(HC)结合后可用作 SIB 阳极材料。合成的碳纳米管具有以下作用:提高阳极材料的导电性、改善充放电循环稳定性和增强钠离子存储。我们通过改变碳纳米管的含量将这些碳纳米管融入硬碳中,用 HC_xCNT(M)表示,其中 x 代表碳纳米管的重量。因此,HC_5CNT(M) 阳极在半电池 SIB 中效果更好,能够处理不同的充电速率,并在 0.2C 速率下达到 256.7 mAh g-1 的最大比容量。这种材料还显示出卓越的稳定性,在 1C 速率下保持 99.48 mAh g-1 的比容量,在 800 次循环后电容保持率仍为 99.1%。根据电化学分析结果,二氧化碳转化的碳纳米管可以提高导电性、比容量和循环稳定性。这种创新方法不仅有助于实现可持续储能,还为减少碳排放和再利用工业废弃物提供了有价值的解决方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
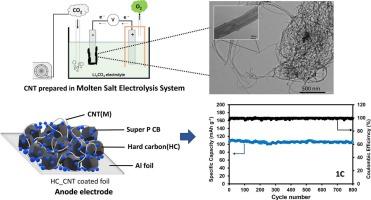
CO2-converted carbon nanotubes produced from molten salt electrolytes process for application of eco-friendly sustainable anode for sodium ion batteries
CO2-converted carbon nanotube (CNT) has been synthesized from industrial carbon dioxide emissions using a molten salt electrochemical process for an eco-friendly sustainable anode of sodium ion batteries (SIBs). The synthesized CNT have diameters ranging from 10 to 100 nm, and they combine with hard carbon (HC) to serve as SIB anode material. The synthesized CNT has the following roles: increasing the electrical conductivity of anode material, improving charge-discharge cycle stability, and enhancing sodium ion storage. We integrate these CNT into hard carbon by varying CNT contents, denoted as HC_xCNT(M), where x represents the weight of the CNT. So, the HC_5CNT(M) anode works better in half-cell SIBs, being able to handle different charging rates and reaching a maximum specific capacity of 256.7 mAh g−1 at a 0.2C-rate. This material also displayed remarkable stability, maintaining a specific capacity of 99.48 mAh g−1 at 1C-rate and the capacitive retention remained at 99.1 % after 800 cycles. The CO2-converted CNT can improve electrical conductivity, specific capacity, and cycle stability, according to electrochemical analysis results. This innovative approach not only contributes to sustainable energy storage but also provides a valuable solution for reducing carbon emissions and repurposing industrial waste.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


