多模态单细胞图谱分析揭示局灶性皮质发育不良的神经元脆弱性和病理细胞状态
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
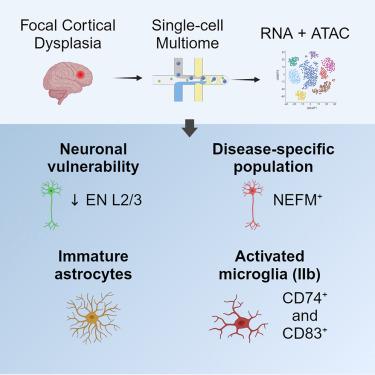
局灶性皮质发育不良(FCD)是一种以大脑皮质畸形为特征的神经发育疾病,常引起耐药性癫痫。在这项研究中,我们进行了多组学单核分析,以绘制 FCD II 型的染色质可及性和转录组图谱,生成了一个全面的多模态单核数据集,其中包括来自 11 个病变和对照临床样本的 61,525 个细胞。我们的发现揭示了影响 FCD 病变中神经元和神经胶质细胞的染色质、转录组和细胞的深刻改变,包括上层兴奋性神经元的选择性缺失、少突胶质细胞和未成熟星形胶质细胞群的显著扩增,以及畸形神经元的独特神经元亚群。此外,我们还发现了活化的小胶质细胞亚群,尤其是在 FCD IIb 病例中。这项全面的研究揭示了驱动 FCD 发展和致痫性的神经元和胶质细胞状态,加深了我们对 FCD 的理解,并为靶向治疗的开发提供了方向。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Multimodal single-cell profiling reveals neuronal vulnerability and pathological cell states in focal cortical dysplasia
Focal cortical dysplasia (FCD) is a neurodevelopmental condition characterized by malformations of the cerebral cortex that often cause drug-resistant epilepsy. In this study, we performed multi-omics single-nuclei profiling to map the chromatin accessibility and transcriptome landscapes of FCD type II, generating a comprehensive multimodal single-nuclei dataset comprising 61,525 cells from 11 clinical samples of lesions and controls. Our findings revealed profound chromatin, transcriptomic, and cellular alterations affecting neuronal and glial cells in FCD lesions, including the selective loss of upper-layer excitatory neurons, significant expansion of oligodendrocytes and immature astrocytic populations, and a distinct neuronal subpopulation harboring dysmorphic neurons. Furthermore, we uncovered activated microglia subsets, particularly in FCD IIb cases. This comprehensive study unveils neuronal and glial cell states driving FCD development and epileptogenicity, enhancing our understanding of FCD and offering directions for targeted therapy development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


