利用荧光生物传感器跟踪肝癌细胞中 1,6-二磷酸果糖的动态变化
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
HYlight 是一种基因编码的荧光生物传感器,可按比例监测 1,6-二磷酸果糖 (FBP),这是一种关键的糖酵解代谢物。鉴于葡萄糖在肝癌代谢中的作用,我们在人类肝癌细胞和原代小鼠肝细胞中表达了 HYlight。通过体外、硅学和细胞内实验,我们发现 HYlight 能够监测与糖酵解而非葡萄糖生成相关的 FBP 变化。HYlight 与 FBP 的亲和力为 1 μM,并且在生理 pH 值范围内稳定。HYlight 与磷酸二羟丙酮的结合力较弱,其比率反应受离子强度和磷酸盐的影响。因此,有必要在体外模拟细胞膜条件,以便在 HYlight 的细胞反应和 FBP 浓度之间建立可靠的相关性。研究发现,FBP 的浓度在较低的微摩尔范围内,远远低于之前的毫摩尔估计值。总之,这种生物传感器方法能以单细胞分辨率实时监测FBP浓度,是了解癌症代谢的宝贵工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
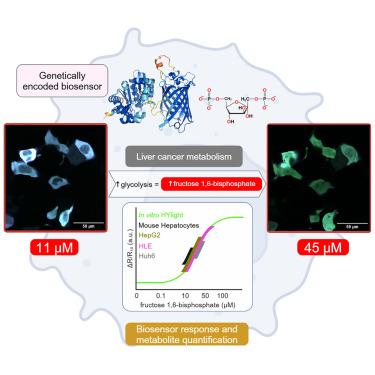
Tracking fructose 1,6-bisphosphate dynamics in liver cancer cells using a fluorescent biosensor
HYlight is a genetically encoded fluorescent biosensor that ratiometrically monitors fructose 1,6-bisphosphate (FBP), a key glycolytic metabolite. Given the role of glucose in liver cancer metabolism, we expressed HYlight in human liver cancer cells and primary mouse hepatocytes. Through in vitro, in silico, and in cellulo experiments, we showed HYlight’s ability to monitor FBP changes linked to glycolysis, not gluconeogenesis. HYlight’s affinity for FBP was ∼1 μM and stable within physiological pH range. HYlight demonstrated weak binding to dihydroxyacetone phosphate, and its ratiometric response was influenced by both ionic strength and phosphate. Therefore, simulating cytosolic conditions in vitro was necessary to establish a reliable correlation between HYlight’s cellular responses and FBP concentrations. FBP concentrations were found to be in the lower micromolar range, far lower than previous millimolar estimates. Altogether, this biosensor approach offers real-time monitoring of FBP concentrations at single-cell resolution, making it an invaluable tool for the understanding of cancer metabolism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


