遗传背景和饮食对小鼠新陈代谢和基因表达的综合影响
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
在人类中,饮食模式对体重和新陈代谢的影响因人而异。为了揭示不同饮食影响的遗传决定因素,我们让四种不同基因的小鼠品系摄入具有相似宏量营养素组成的人源化饮食(美式、地中海式、素食和纯素),并进行了体重、代谢参数和 RNA-seq 分析。我们观察到饮食和品系对体重、甘油三酯和胰岛素水平的明显影响。脂肪量、脂肪组织和骨骼肌葡萄糖摄取量的差异,以及大多数组织中基因表达的变化都与菌株有关。在内脏脂肪组织中,有400多个基因以菌株依赖性的方式对饮食做出反应,其中许多是代谢物转运和脂质代谢途径中的基因,有几个基因先前已被确认会改变人类的饮食效应。因此,遗传背景对新陈代谢有深远影响,尽管选择的饮食模式会改变强烈的遗传效应。这项研究为今后在小鼠中开展菌株-饮食相互作用的机理研究以及将其转化为人类的精准营养铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
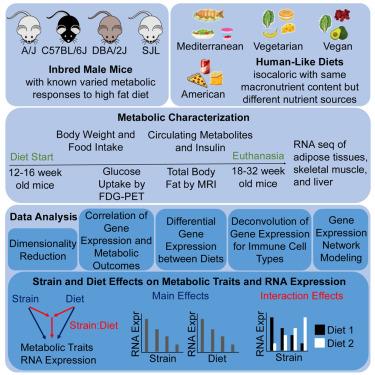
Combined effects of genetic background and diet on mouse metabolism and gene expression
In humans, dietary patterns impact weight and metabolism differentially across individuals. To uncover genetic determinants for differential dietary effects, we subjected four genetically diverse mouse strains to humanized diets (American, Mediterranean, vegetarian, and vegan) with similar macronutrient composition, and performed body weight, metabolic parameter, and RNA-seq analysis. We observed pronounced diet- and strain-dependent effects on weight, and triglyceride and insulin levels. Differences in fat mass, adipose tissue, and skeletal muscle glucose uptake, and gene expression changes in most tissues were strain-dependent. In visceral adipose tissue, ∼400 genes responded to diet in a strain-dependent manner, many of them in metabolite transport and lipid metabolism pathways and several previously identified to modify diet effects in humans. Thus, genetic background profoundly impacts metabolism, though chosen dietary patterns modify the strong genetic effects. This study paves the way for future mechanistic investigations into strain-diet interactions in mice and translation to precision nutrition in humans.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


