可能来自肿瘤内微生物的免疫原肽:结直肠癌治疗的机遇
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
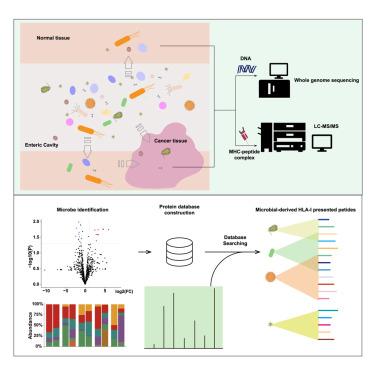
最近的证据证实了肿瘤内微生物的存在,但它们对免疫肽组的影响在很大程度上仍未得到探索。在这里,我们采用了一种综合策略来鉴定源自肿瘤内微生物的免疫肽组。通过分析 10 名结直肠癌(CRC)患者,我们确定了 154 种来源于微生物的人类白细胞抗原(HLA)-I 配体。这些肽主要来源于细菌,特别是在核酸镰刀菌(Fusobacterium nucleatum)中含量丰富,核酸镰刀菌是区分正常组织和肿瘤组织最普遍的细菌。我们发现了源自核酸镰刀菌的 20 种肽,其中 13 种(包括多种患者共有的两种肽)具有肿瘤特异性。验证实验证实,推测的微生物衍生肽可激活 CD8+ T 细胞反应。我们的研究结果表明,HLA-I分子能够呈现肿瘤内微生物衍生的肽,可能有助于CD8+ T细胞介导的免疫,并为癌症免疫疗法提出了潜在的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Immunogenic peptides putatively from intratumor microbes: Opportunities for colorectal cancer treatment
Recent evidence has confirmed the presence of intratumor microbes, yet their impact on the immunopeptidome remains largely unexplored. Here we introduced an integrated strategy to identify the immunopeptidome originated from intratumor microbes. Analyzing 10 colorectal cancer (CRC) patients, we identified 154 putative microbe-derived human leukocyte antigen (HLA)-I ligands. Predominantly bacterial in origin, these peptides were notably abundant in Fusobacterium nucleatum, the most prevalent bacterium differentiating between normal and tumor tissues. We discovered 20 peptides originating from F. nucleatum, thirteen of which, including two peptides shared across multiple patients, were tumor specific. Validation experiments confirmed that the putative microbe-derived peptide could activate CD8+ T cell responses. Our findings indicate that HLA-I molecules are capable of presenting intratumor microbe-derived peptides in CRC, potentially contributing to CD8+ T cell-mediated immunity and suggesting potential strategies for cancer immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


