多光子成像技术用于量化植入大鼠颅骨缺损后 PEG 颗粒水凝胶支架中间充质干细胞的存活和分布情况
引用次数: 0
摘要
间充质干细胞(MSCs)具有分泌特性和成骨细胞分化能力,是促进骨再生的细胞疗法的理想候选者。然而,通过注射输送的间充质干细胞通常有< 5% 会在输送后几天内滞留在愈合部位。这项研究利用颗粒状 PEG 水凝胶支架将标记有荧光量子点的间充质干细胞输送到临界大小的大鼠腓骨缺损处。在植入后 3 天和 7 天,用多光子显微镜评估了间充质干细胞在水凝胶支架内的存在、存活和分布情况。此外,还对渗入支架的内源性细胞进行了量化,并通过免疫组化鉴定了 M1 和 M2 巨噬细胞的标记。这种多光子显微镜技术能定量分析外源性间充质干细胞的存在和存活情况,并能以微米级的空间分辨率观察细胞在整个植入支架中的分布情况。通过PEG颗粒水凝胶支架将75万个间充质干细胞植入腓骨缺损处,植入后3天和7天存活率分别为27%和8%。植入后 3 天和 7 天,外源性间充质干细胞和浸润的内源性细胞(包括 M1 和 M2 巨噬细胞)在整个支架上分布均匀。这种多光子显微镜技术可用于评估生物材料输送系统,以提高外源性间充质干细胞的存在和存活率,促进内源性细胞浸润,并研究外源性细胞与内源性细胞之间的相互作用,从而促进骨再生疗法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
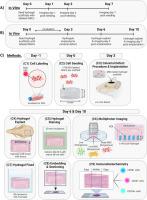
Multiphoton imaging for quantification of mesenchymal stem cell survival and distribution in PEG granular hydrogel scaffolds post-implantation into rat cranial bone defects
Mesenchymal stem cells (MSCs) are promising candidates for cellular therapies aimed at promoting bone regeneration due to their secretory properties and osteoblastic differentiation capacity. However, typically < 5% of delivered MSCs are retained at the healing site within days of delivery via injection. In this work, granular PEG hydrogel scaffolds were used to deliver MSCs, labeled with fluorescent Quantum Dots, into critical-sized rat calvarial bone defects. The presence, survival, and distribution of MSCs within the hydrogel scaffold were evaluated with multiphoton microscopy at 3- and 7-days post-implantation. Additionally, endogenous cell infiltration into scaffolds was quantified, and markers for M1 and M2 macrophages were identified with immunohistochemistry. This multiphoton microscopy technique provides a quantitative analysis of exogenous MSC presence and survival and allows for micron-level spatial resolution of cell distribution throughout the implanted scaffolds. When ∼750,000 MSCs were implanted in a calvarial bone defect via PEG granular hydrogel scaffolds, ∼27% and ∼8% survived 3- and 7-days post-implantation, respectively. At 3- and 7-days post-implantation, exogenous MSCs and infiltrating endogenous cells, including M1 and M2 macrophages, were well distributed throughout the scaffolds. This multiphoton microscopy technique could be used to assess biomaterial delivery systems that can improve exogenous MSC presence and survival, facilitate endogenous cell infiltration, and investigate exogenous-endogenous cell interactions for bone regeneration therapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomedical engineering advances
Bioengineering, Biomedical Engineering
自引率
0.00%
发文量
0
审稿时长
59 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


