通过第一原理筛选和 d 波段中心控制设计的囊泡状 Co-Ni-S 可用于主动氢气进化
IF 6.7
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
氢进化反应(HER)被认为是制氢的有效途径。为了获得高效的 HER 催化剂,本文采用密度泛函理论找出硫化镍的最佳候选物。在机理筛选的指导下,一步水热法合成了高活性 HER 电催化剂 Co 取代 Ni3S4。膜上的裂缝为反应物分子进入囊泡提供了合理的入口。此外,内在活性的增强还归因于 d 波段中心的上移。从反应能量图来看,H2O* 的分解被确认为速率决定步骤(RDS),而 RDS 所需的能量因 d 带中心的升高而有效降低,这使得催化剂在 100 mA-cm-2 时的过电位达到 185 mV,在 20 mA-cm-2 时的过电位比最大 Co-Ni-S 相对电催化剂低 38 mV。本文章由计算机程序翻译,如有差异,请以英文原文为准。
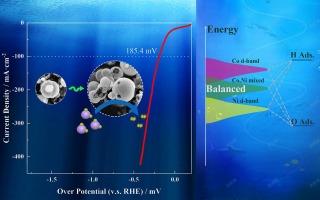
Vesicle-Shaped Co-Ni-S designed by first principles screening and d-Band center control for active hydrogen evolution
Hydrogen evolution reaction (HER) is considered as an effective pathway for hydrogen production. Herein, to obtain efficient HER catalyst, density functional theory is employed to figure out the optimal candidate for nickel sulfide. Guided by the mechanistic screening, the highly active HER electrocatalyst, Co-substituted Ni3S4, is synthesized by one-step hydrothermal method, resembling a vesicle composed of a broken spherical membrane and encapsulated clusters of nanospheres. Cracks on membrane provide a rational entry for reactant molecules to be enclosed in the vesicle. Additionally, the enhanced intrinsic activity also attributes to the upward shift of the d-band center. From the reaction energy diagrams, the decomposition of H2O* is confirmed as the rate-determining step (RDS) and the energy required for the RDS is efficiently lowered by the elevated d-band center, which made the catalyst achieves 185 mV overpotential at 100 mA·cm−2 and 38 mV at 20 mA·cm−2 lower than utmost Co-Ni-S relative electrocatalyst.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


