用氘同位素示踪法定量研究煤直接液化过程中的氢活化机理
IF 6.7
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
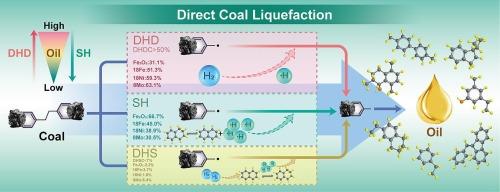
了解氢活化对于提高煤直接液化(DCL)的性能至关重要,而煤直接液化是将煤清洁、高效地转化为燃料油和芳香化学品的关键。煤直接液化涉及三种氢源:溶剂氢(SH)、直接反应的溶解氢(DHD)和通过溶剂反应的溶解氢(DHS)。定量研究 DHS 具有挑战性,因为它是通过气相 H2 和溶剂加氢生成的氢供体溶剂的脱氢反应产生的。这项研究首次根据同位素示踪剂反应后溶剂的氕和氘核磁共振结果,建立了确定 DHS 消耗量 (DHSC) 的定量方法。为了证实该方法的可靠性,还进行了对比实验和量子化学计算。同位素示踪法表明,在硬脂酸铁、硬脂酸镍或 2- 乙基己酸钼催化下,DHSC 占氢气总消耗量的 7%,而 DHD 占 50%。因此,DHD 而不是 DHS 是催化活化的主要氢源。此外,对比实验还表明,单一氢源的氢消耗量大于三种氢源共存时的氢消耗量,这表明三种氢源之间存在竞争。量子化学计算表明,三种氢源之间的竞争顺序为 DHS < SH < DHD,这与同位素示踪实验中氢气消耗的顺序一致。该研究定量揭示了催化剂活化氢气的机理,为溶剂和催化剂的优化和相互匹配提供了科学依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Using isotope-tracer method with deuterium for quantitative study of hydrogen activation mechanism in direct coal liquefaction
Understanding hydrogen activation is crucial for improving the performance of direct coal liquefaction (DCL) which is essential for the clean, efficient conversion of coal to fuel oils and aromatic chemicals. Three hydrogen sources are involved in DCL: solvent hydrogen (SH), dissolved hydrogen that reacts directly (DHD), and dissolved hydrogen that reacts through a solvent (DHS). Quantitatively studying DHS is challenging because it is generated through the dehydrogenation of hydrogen-donor solvents produced via the hydrogenation of gas-phase H2 and solvents. This research establishes for the first time a quantitative method for determining DHS consumption (DHSC) based on protium- and deuterium-nuclear-magnetic-resonance results for solvents after isotope-tracer reactions. Comparative experiments and quantum-chemical calculations were performed to confirm the method’s reliability. The isotope-tracer method showed that DHSC accounts for < 7 % of the total hydrogen consumption under catalysis with ferric stearate, nickel stearate, or molybdenum 2-ethylhexanoate, while DHD consumption accounts for > 50 %. Thus, DHD, rather than DHS, is the primary hydrogen source for catalytic activation. Furthermore, the comparative experiments also showed that hydrogen consumption is greater for one hydrogen source than for the coexistence of the three hydrogen sources, indicating competition among the three sources. The quantum-chemical calculations showed that the competitiveness among the three sources follows the order of DHS < SH < DHD, this agrees with the order of hydrogen consumption in the isotope-tracer experiments. This study quantitatively reveals the mechanism responsible for hydrogen activation by catalysts and provides a scientific basis for optimization and mutual matching of solvents and catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


