利用掺铁磷化镍进行光热增强型电催化水分离
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
尽管电催化水分离在制氢方面前景广阔,但不利的反应能垒和动力学特性导致转化效率不尽人意。在此,我们提供了一种创新策略,通过近红外(NIR)诱导的光热效应来优化 Fe/Ni2P 催化剂的电化学活性。在氢进化反应(HER)和氧进化反应(OER)的超低过电位(分别为 16 mV 和 167 mV)下,Fe/Ni2P-NIR 的电流密度分别为 10 mA cm-2,塔菲尔斜率分别为 38.7 和 46.2 mV dec-1。这种双功能催化剂还能在 1.40 V 的低电压下提供 10 mA cm-2 的电流,用于整体水分离。近红外光诱导的局部热效应激活了丰富的催化位点,加速了电荷和质量的转移,并改善了内在反应动力学。在密度泛函理论(DFT)计算的指导下,光热效应降低了HER过程中Fe/Ni2P上*H解吸和OER过程中其重构活性相NiFeOOH上*O形成的速率决定步骤(RDS)的能量障碍。我们在阴离子交换膜(AEM)电解槽中实现了与 Fe/Ni2P-NIR 的光热-电化学集成,在 1.76 V 电压下可达到 500 mA cm-2,并在 50 h 内具有极佳的稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
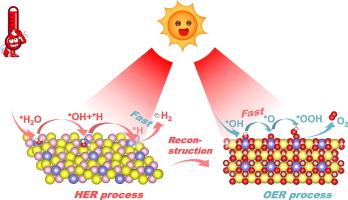
Photothermally enhanced electrocatalytic water splitting with iron-doped nickel phosphide
Although electrocatalytic water splitting holds significant promise for hydrogen production, unfavorable reaction energy barriers and kinetic properties lead to unsatisfactory conversion efficiency. Herein, we provide an innovative strategy to optimize the electrochemical activity of the Fe/Ni2P catalyst through near-infrared (NIR)-induced photothermal effect. The Fe/Ni2P-NIR yields a current density of 10 mA cm−2 at ultralow overpotentials of 16 mV for the hydrogen evolution reaction (HER) and 167 mV for the oxygen evolution reaction (OER), with Tafel slopes of 38.7 and 46.2 mV dec−1, respectively. This bifunctional catalyst also delivers 10 mA cm−2 at a low voltage of 1.40 V for overall water splitting. The NIR photoinduced local thermal effect activates abundant catalytic sites, accelerates charge and mass transfer, and improves intrinsic reaction kinetics. Guided by density functional theory (DFT) calculations, the photothermal effect reduces the energy barriers of the rate-determining steps (RDS) for *H desorption on Fe/Ni2P during HER and *O formation on its reconstructed active phase NiFeOOH during OER. We realized photothermal-electrochemical integration with Fe/Ni2P-NIR in an anion exchange membrane (AEM) electrolyzer, attaining 500 mA cm−2 at 1.76 V, with excellent stability over 50 h. This strategy may significantly advance energy conversion technology towards economic hydrogen production through water electrolysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


