键工程:弱化 Ru-O 共价关系,在酸性溶液中实现高效稳定的水氧化作用
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
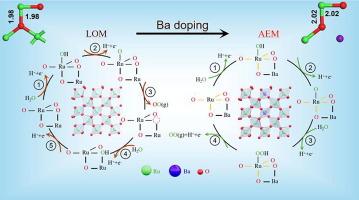
二氧化钌(RuO2)在酸性氧进化反应(OER)中的持久稳定性受到 OER 过程中晶格氧(LO)和金属溶解的影响。掺杂异原子被认为是提高二氧化钌在酸性 OER 应用中稳定性的可行策略。本研究在 RuO2(Ba-RuO2)纳米片(NS)催化剂中掺入了不易得失电子的离子 Ba2+,从而增加了暴露的活性位点数量,使电流密度达到 10 mA/cm2,过电位仅为 229 mV,并能维持 250 小时以上。这种变化减少了 LO 的参与和钌(Ru)的溶解,从而显著提高了催化剂的长期耐久性。此外,衰减全反射-表面增强红外吸收光谱分析证实,由于掺杂了 Ba,OER 机制从 LO 介导的途径转变为吸附剂演化途径,从而避免了 Ru 的过氧化,进一步提高了 RuO2 的稳定性。此外,DFT 研究结果还发现,掺杂 Ba 能优化中间产物的吸附能,从而提高 OER 在酸性环境中的活性。这项研究提供了一种有效的策略,可用于指导未来开发基于 Ru 的氧化物催化剂在酸性环境中的稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Bond engineering: weakening Ru–O covalency for efficient and stable water oxidation in acidic solutions
The persistent stability of ruthenium dioxide (RuO2) in acidic oxygen evolution reactions (OER) is compromised by the involvement of lattice oxygen (LO) and metal dissolution during the OER process. Heteroatom doping has been recognized as a viable strategy to foster the stability of RuO2 for acidic OER applications. This study presented an ion that does not readily gain or lose electrons, Ba2+, into RuO2 (Ba–RuO2) nanosheet (NS) catalyst that increased the number of exposed active sites, achieving a current density of 10 mA/cm2 with an overpotential of only 229 mV and sustaining this output for over 250 h. According to density functional theory (DFT) and X-ray absorption spectroscopy, Ba doping resulted in a longer Ru–O bond length, which in turn diminished the covalency of the bond. This alteration curtailed the involvement of LO and the dissolution of ruthenium (Ru), thereby markedly improving the durability of the catalyst over extended periods. Additionally, attenuated total reflectance-surface enhanced infrared absorption spectroscopy analysis substantiated that the OER mechanism shifted from a LO-mediated pathway to an adsorbate evolution pathway due to Ba doping, thereby circumventing Ru over-oxidation and further enhancing the stability of RuO2. Furthermore, DFT findings uncovered that Ba doping optimizes the adsorption energy of intermediates, thus enhancing the OER activity in acidic environments. This study offers a potent strategy to guide future developments on Ru-based oxide catalysts’ stability in an acidic environment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


