抑制锌-氧化物水电池中不可逆的 Zn2+/H+ 共插入化学反应以提高容量稳定性
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
可充电含水锌氧化物电池是一种前景广阔的储能设备,具有理论比容量高、成本低的特点。然而,在传统电解质中,MoO3 阴极在初始放电/充电过程中容量急剧下降,导致循环寿命短,给 Zn-MoOx 电池的开发带来了挑战。在此,我们全面研究了 MoO3 阴极的溶解机制,并创新性地引入了一种聚合物来抑制不可逆过程。我们的研究结果表明,这种容量衰减源于水性电解质中不可逆的 Zn2+/H+ 共掺杂/萃取过程。更糟糕的是,在 Zn2+ 插层过程中,所形成的具有较低价态(Mo5+/Mo4+)的 ZnxMoO3-x 中间相在水环境中会发生严重溶解。为了应对这些挑战,我们首次在 MoO3 纳米棒周围涂覆了一层聚苯胺(PANI)外壳,有效地抑制了这些不可逆过程,并在长期循环过程中保护了结构的完整性。详细的结构分析和理论计算表明,PANI@MoO3-x 中的 =N- 基团同时削弱了对 H+ 的吸附并增强了对 Zn2+ 的吸附,从而使 PANI@MoO3-x 阴极具有可逆的 Zn2+/H+ 插层/萃取能力。因此,所获得的 PANI@MoO3-x 阴极在 0.1 A g-1 的条件下可提供 316.86 mA h g-1 的出色放电容量,并且在 5 A g-1 条件下循环 1000 次后仍能保持 75.49% 的容量。这项研究解决了与 MoO3 阴极相关的关键问题,极大地推动了对 Zn-MoO3 水电池中竞争性多离子储能机制的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
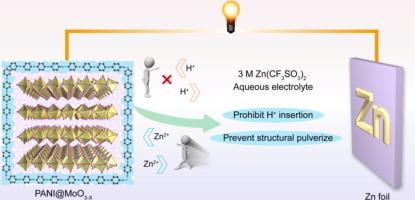
Inhibiting irreversible Zn2+/H+ co-insertion chemistry in aqueous zinc-MoOx batteries for enhanced capacity stability
Rechargeable aqueous Zn-MoOx batteries are promising energy storage devices with high theoretical specific capacity and low cost. However, MoO3 cathodes suffer drastic capacity decay during the initial discharging/charging process in conventional electrolytes, resulting in a short cycle life and challenging the development of Zn-MoOx batteries. Here we comprehensively investigate the dissolution mechanism of MoO3 cathodes and innovatively introduce a polymer to inhibit the irreversible processes. Our findings reveal that this capacity decay originates from the irreversible Zn2+/H+ co-intercalation/extraction process in aqueous electrolytes. Even worse, during Zn2+ intercalation, the formed ZnxMoO3−x intermediate phase with lower valence states (Mo5+/Mo4+) experiences severe dissolution in aqueous environments. To address these challenges, we developed a first instance of coating a polyaniline (PANI) shell around the MoO3 nanorod effectively inhibiting these irreversible processes and protecting structural integrity during long-term cycling. Detailed structural analysis and theoretical calculations indicate that =N– groups in PANI@MoO3−x simultaneously weaken H+ adsorption and enhance Zn2+ adsorption, which endowed the PANI@MoO3−x cathode with reversible Zn2+/H+ intercalation/extraction. Consequently, the obtained PANI@MoO3−x cathode delivers an excellent discharge capacity of 316.86 mA h g−1 at 0.1 A g−1 and prolonged cycling stability of 75.49% capacity retention after 1000 cycles at 5 A g−1. This work addresses the critical issues associated with MoO3 cathodes and significantly advances the understanding of competitive multi-ion energy storage mechanisms in aqueous Zn-MoO3 batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


