一种具有多电子氧化还原位点的供体-受体共轭双极聚合物,适用于长循环寿命和高倍率水性锌双离子电池
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
锌离子水电池(AZIB)因其与生俱来的安全性、合理的价格和可持续性,在大规模储能方面具有巨大的潜在优势。然而,大多数 AZIB 正极材料都存在循环寿命短和速率性能差的问题。本文开发了一种双极供体-受体(D-A)共轭微孔聚合物(PTZ-BDTB),它由电子吸收苯并[1,2-b:4,5-b']二噻吩-4,8-二酮(BDTB)单元和电子供体吩噻嗪(PTZ)单元组成,可作为水性锌双离子电池(AZDIB)的阴极材料。D-A 型结构设计可以减小带隙,从而促进聚合物框架中的电子转移。因此,在 30 mol/kg (m) ZnCl2 水包盐电解液中,PTZ-BDTB 阴极在 0.05 A g-1 电流条件下显示出 202 mA h g-1 的高可逆容量和优异的速率性能(在 15 A g-1 电流条件下为 109 mA h g-1),远远优于同类聚合物 PPTZ 和 PB-BDTB。令人印象深刻的是,PTZ-BDTB 具有超稳定的循环性能,在 0.05 A g-1 条件下循环 460 次后容量保持率为 76.2%,在 5 A g-1 条件下循环 27000 次后容量保持率为 96%。PTZ-BDTB 还表现出较低的自放电能力,电池静置 28 天后容量保持率约为 76.4%。这些结果表明,D-A 型结构设计是构建高性能 AZDIB 正极材料的一种有前途的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
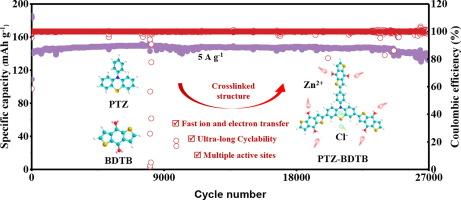
A donor-acceptor conjugated bipolar polymer with multielectron redox sites for long-cycle-life and high-rate aqueous zinc dual-ion batteries
Aqueous zinc-ion batteries (AZIBs) have hugely latent advantages in large-scale energy storage due to its innate safety, reasonable price, and sustainability. However, most AZIB cathode materials suffer from short cycling life and poor rate performance. Herein, a bipolar donor-acceptor (D-A) conjugated microporous polymer (PTZ-BDTB), consisting of electron-withdrawing benzo[1,2-b:4,5-b’]dithiophene-4,8-dione (BDTB) units and electron-donating phenothiazine (PTZ) units, is developed as the cathode material of aqueous zinc dual-ion batteries (AZDIBs). The D-A type structure design could reduce the band gap, thus promoting electron transfer in the polymer framework. Therefore, the PTZ-BDTB cathode in a 30 mol/kg (m) ZnCl2 water-in-salt electrolyte exhibits a high reversible capacity of 202 mA h g−1 at 0.05 A g−1 with excellent rate performance (109 mA h g−1 at 15 A g−1), which is far superior to its counterpart polymers PPTZ and PB-BDTB. Impressively, PTZ-BDTB shows ultra-stable cycle performance with capacity retention ratios of 76.2% after 460 cycles at 0.05 A g−1 and 96% after 27000 cycles at 5 A g−1. PTZ-BDTB also exhibits a low self-discharge ability with capacity retention about 76.4% after resting the battery for 28 days. These results demonstrate that D-A type structural design is a promising strategy for constructing high performance cathode materials for AZDIBs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


