开发具有同时改善稳定性和非天然底物活性的工程糖氨基转移酶,以合成葡萄糖苷酶抑制剂缬烯胺
IF 10.1
1区 工程技术
Q1 ENGINEERING, MULTIDISCIPLINARY
引用次数: 0
摘要
糖胺转移酶(SAT)可催化手性胺与特定酮糖的结合,产生具有生物活性的氨基糖。它们的活性已被用于人工反应,例如利用 SAT WecE 将缬烯酮转化为有价值的 α-葡萄糖苷酶抑制剂缬烯胺。然而,由于其热稳定性低,对非天然底物的活性有限,阻碍了它们的应用。由于稳定性和活性之间存在公认的固有权衡,因此同时提高稳定性和酶活性尤其具有挑战性。为了同时提高 WecE 对缬烯酮的稳定性和活性,我们采用了一种定制的组合活性位点饱和试验-迭代饱和诱变(CAST-ISM)策略。根据对 WecE 活性位点 57 个残基的进化保护和平均突变折叠能评估,确定了 14 个与提高稳定性和活性权衡有关的热点。通过定点饱和诱变(SSM)和迭代进化循环完成了这些特定残基的正突变和组合突变。与野生型(WT)WecE 相比,四重突变体 M4(Y321F/K209F/V318R/F319V)在 40 °C 时的半衰期(t1/2)延长了 641.49 倍,对非天然底物缬烯酮的活性提高了 31.37 倍。三重突变体 M3(Y321F/K209F/V318R)在 40 °C 时的半衰期(t1/2)延长了 83.04 倍,对缬烯酮的活性提高了 37.77 倍。与 WT 相比,突变体的界面相互作用增强,转氨酶反应催化距离缩短,从而提高了突变体的稳定性和活性。因此,我们完成了一种以目标为导向的通用策略,为人工氨基酸生物合成应用获得了稳定和高活性的 SATs。本文章由计算机程序翻译,如有差异,请以英文原文为准。
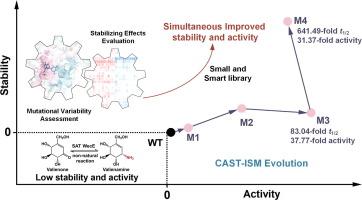
Development of an Engineered Sugar Aminotransferase with Simultaneously Improved Stability and Non-Natural Substrate Activity to Synthesize the Glucosidase Inhibitor Valienamine
Sugar aminotransferases (SATs) catalyze the installation of chiral amines onto specific keto sugars, producing bioactive amino sugars. Their activity has been utilized in artificial reactions, such as using the SAT WecE to transform valienone into the valuable α-glucosidase inhibitor valienamine. However, the low thermostability and limited activity on non-natural substrates have hindered their applications. Simultaneously improving stability and enzyme activity is particularly challenging owing to the acknowledged inherent trade-off between stability and activity. A customized combinatorial active-site saturation test–iterative saturation mutagenesis (CAST-ISM) strategy was used to simultaneously enhance the stability and activity of WecE toward valienone. Fourteen hotspots related to improving the stability–\activity trade-off were identified based on evolutionary conservation and the average mutation folding energy assessment of 57 residues in the active site of WecE. Positive mutagenesis and combinatorial mutations of these specific residues were accomplished via site-directed saturation mutagenesis (SSM) and iterative evolution cycles. Compared with those of the wild-type (WT) WecE, the quadruple mutant M4 (Y321F/K209F/V318R/F319V) displayed a 641.49-fold increase in half-life (t1/2) at 40 °C and a 31.37-fold increase in activity toward the non-natural substrate valienone. The triple mutant M3 (Y321F/K209F/V318R) demonstrated an 83.04-fold increase in (t1/2) at 40 °C and a 37.77-fold increase in activity toward valienone. The underlying mechanism was dependent on the strengthened interface interactions and shortened transamination reaction catalytic distance, compared with those of the WT, which improved the stability and activity of the obtained mutants. Thus, we accomplished a general target-oriented strategy for obtaining stable and highly active SATs for artificial amino-sugar biosynthesis applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Engineering
Environmental Science-Environmental Engineering
自引率
1.60%
发文量
335
审稿时长
35 days
期刊介绍:
Engineering, an international open-access journal initiated by the Chinese Academy of Engineering (CAE) in 2015, serves as a distinguished platform for disseminating cutting-edge advancements in engineering R&D, sharing major research outputs, and highlighting key achievements worldwide. The journal's objectives encompass reporting progress in engineering science, fostering discussions on hot topics, addressing areas of interest, challenges, and prospects in engineering development, while considering human and environmental well-being and ethics in engineering. It aims to inspire breakthroughs and innovations with profound economic and social significance, propelling them to advanced international standards and transforming them into a new productive force. Ultimately, this endeavor seeks to bring about positive changes globally, benefit humanity, and shape a new future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


