利用液态金属合金催化剂在室温下将二氧化碳转化为碳,无需外部能量输入
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
使用催化剂将二氧化碳转化为碳通常需要大量的能量输入,如热量、压力或电力。这是因为二氧化碳的键很强,启动反应需要能量。如果使用化石燃料,二氧化碳转化所需的能量还可能导致碳排放。在低温和没有外部能量的情况下,二氧化碳转化为固态碳的探索较少。在此,我们展示了一种液态金属合金(In0.2Ga0.8)作为催化剂,可在室温下将二氧化碳转化为碳,而无需外部能量。对形成的碳进行了表征,计算得出的还原反应自由能为 -530 kJ mol-1。在陶瓷表面涂覆的催化剂在室温下也观察到了类似的二氧化碳转化为碳的现象。催化剂涂层陶瓷膜的转化率是块状催化剂的五倍。在室温下二氧化碳连续流动时,催化膜的二氧化碳转化效率为 60%。EDX 和 XPS 研究证实了催化剂表面碳的形成。我们的研究可能会开启膜催化二氧化碳的话题,在工业规模上以较低的运营成本实现实际的碳转化,从而实现净零排放。本文章由计算机程序翻译,如有差异,请以英文原文为准。
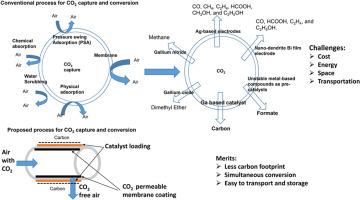
Room-temperature CO2 conversion to carbon using liquid metal alloy catalysts without external energy input
Conversion of CO2 to carbon using a catalyst typically requires significant energy input such as heat, pressure, or electricity. This is due to its strong bonds and the energy needed to initiate the reaction. The energy requirement for CO2 conversion may also lead to carbon emissions if sources are fossil-based. There is less exploration at low temperatures and without an external energy CO2 conversion to solid carbon. Here, we show a liquid metal alloy (In0.2Ga0.8) used as a catalyst for converting CO2 to carbon at room temperature without external energy. The carbon formed was characterized, and the calculated free energy of the reduction reaction was −530 kJ mol−1.The catalyst coated over a ceramic surface observed similar phenomena of CO2 conversion to carbon at room temperature. The conversion was five times higher at catalyst-coated ceramic membranes than at bulk catalysts. The CO2 conversion efficiency was 60 % at the catalytic membrane for continuous flow of CO2 at room temperature. EDX and XPS studies confirmed the formation of carbon at the catalyst surface. Our study may open the topic of membrane-based catalysis of CO2 for practical carbon conversion at lower operating costs at the industrial scale to achieve net-zero emissions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


