MIL-53 对茂金属在丙烯聚合过程中催化性能的影响
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在本研究中,我们探讨了金属有机框架 (MOF) 作为茂金属催化剂载体在丙烯聚合中的应用。我们采用 MIL-53 等 MOFs 以及 SBA-15 和 SiO2 等其他传统载体来固定用甲基铝氧烷 (MAO) 活化的 Me2Si(2-Me-4-PhInd)2ZrCl2 催化剂。研究了这些支撑物的物理化学特性对催化性能和聚合物特性的影响。以通道结构为特征的 MIL-53 有助于生产分子量更高的聚丙烯,其催化活性是 SBA-15 和 SiO2 载体的两倍。MIL-53/MAO/Me2Si(2-Me-4-PhInd)2ZrCl2 催化剂还表现出更高的共聚单体掺入率和更低的聚合物熔点。MIL-53/MAO/Me2Si(2-Me-4-PhInd)2ZrCl2 催化剂的活性更高、分子量更大、对 1-hexene 的反应活性更强,这些都归功于 MIL-53 的电子和立体效应。这项研究强调了 MOF 结构在影响聚合行为方面的重要作用,并凸显了 MOF 支持的催化剂在定制聚合物特性方面的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
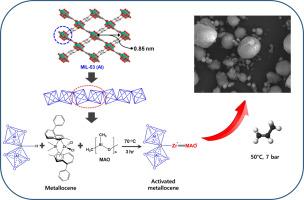
Influence of MIL-53 on the catalytic performance of metallocene in propylene polymerization
In this study, we investigate the application of metal-organic frameworks (MOFs) as a support for metallocene catalysts in the polymerization of propylene. MOFs such as MIL-53 and other conventional supports, SBA-15 and SiO2, were employed to immobilize the Me2Si(2-Me-4-PhInd)2ZrCl2 catalyst activated with methylaluminoxane (MAO). The effects of the physicochemical properties of these supports on catalytic performance and polymer properties were investigated. MIL-53, characterized by its channel structure, facilitated higher molecular weight polypropylene production and exhibited double the catalytic activity of SBA-15 and SiO2 supports. The MIL-53/MAO/Me2Si(2-Me-4-PhInd)2ZrCl2 catalyst also demonstrated higher comonomer incorporation and lower polymer melting points. The higher activity, elevated molecular weight, and increased reactivity towards 1-hexene of the MIL-53/MAO/Me2Si(2-Me-4-PhInd)2ZrCl2 catalyst are attributed to the electronic and steric effects of MIL-53. This study underscores the significant role of MOF structure in influencing polymerization behavior and highlights the potential of MOF-supported catalysts for tailored polymer properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


